You need to know about the UK becoming the first to approve treatments for diseases
The Casgevy treatment for sickle-cell disease in patients with recurrent pain crises requires approval from the UK Medicines and healthcare products regulatory agency
For patients with the most serious form of hemoglobinopathies, as well as those with recurrent pain crises, and for 12 years and older, the authorization from the UK Medicines and healthcare products regulatory agency is necessary. The therapy is offered to a number of people in the UK.
David Rueda, geneticist at Imperial College London, told the SMC that it is well known that CRISPR can result in spurious genetic modifications with unknown consequences. The whole-genome data for these cells would need to be looked at before a conclusion could be reached. Nonetheless, this announcement makes me feel cautiously optimistic.”
The UK agency greenlit the groundbreaking treatment after “a rigorous assessment of its safety, quality and effectiveness,” it said in a statement. The approval must be granted for one year. Vertex and Crispr Therapeutics will need to provide more data on the therapy’s safety and efficacy for it to remain available.
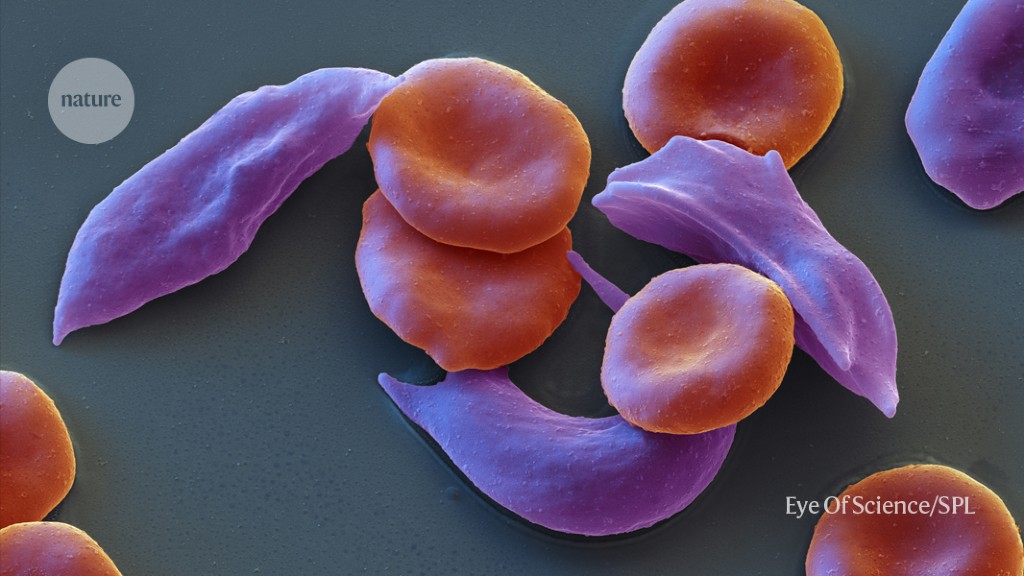
Sickle-cell disease and beta-thalassaemia are caused by errors in the DNA sequence of genes that encode for haemoglobin, a molecule that helps red blood cells to carry oxygen around the body.
29 out of 45 participants have been followed in the trial and results have been drawn. Casgevy completely relieved 28 of those people of debilitating episodes of pain for at least one year after treatment.
The treatment for a severe form of the disease is usually done once a month. In this trial, 54 participants received Casgevy and 42 patients have participated for long enough to provide interim results. At least 39 participants did not need a red-blood-cell transfusion at the one year mark. The need for blood transfusions for three people was slashed by more than 70%.
A Study of a Novel Blood Stem Cell Therapy for Pain and Fluctuations in Low- and Middle-Income Countries
Blood cells that are sticky are more prone to clumping and can cause problems with the blood vessels. Pain crises can be caused by the reduced oxygen supply to tissues.
It’s a disorder in which there is a deficiency of haemoglobin in red blood cells, low numbers of red blood Cells, and symptoms such as irregular heartbeats and fatigue.
For now, the therapy is likely to remain the reserve of rich nations with developed health-care systems. “This treatment may not easily scale up to be able to provide treatments in low- and middle-income countries, since it requires the technology to obtain a patient’s blood stem cells, deliver the genetic editor to these stem cells, and then reinjection of these cells,” geneticist Simon Waddington at University College London told the SMC. He claims that it is not an off the shelf medicine that can be taken with a pill.
Participants involved in the trials, which are ongoing, experienced side effects including nausea, fatigue, fever and an increased risk of infection, but no significant safety concerns were identified. The MHRA and manufacturer are monitoring the safety of the technology and will release further results.
According to estimates, the treatment could cost between US$2 million and US$2 million a patient in the United Kingdom, in line with the prices of other gene therapies.
“We have not established a list price for the UK at this time and are focused on working with the health authorities to secure reimbursement and access for eligible patients as quickly as possible,” a Vertex spokesperson told Nature.

