The transmutation of carbon-to-nitrogen was done by azaarenes
The necessary nitrogen atom: a versatile, versatile design element for biomedical synthesis. The case of quinolines in pharmaceuticals (J. Med. Chem. 62, 3552-3579 (2018))
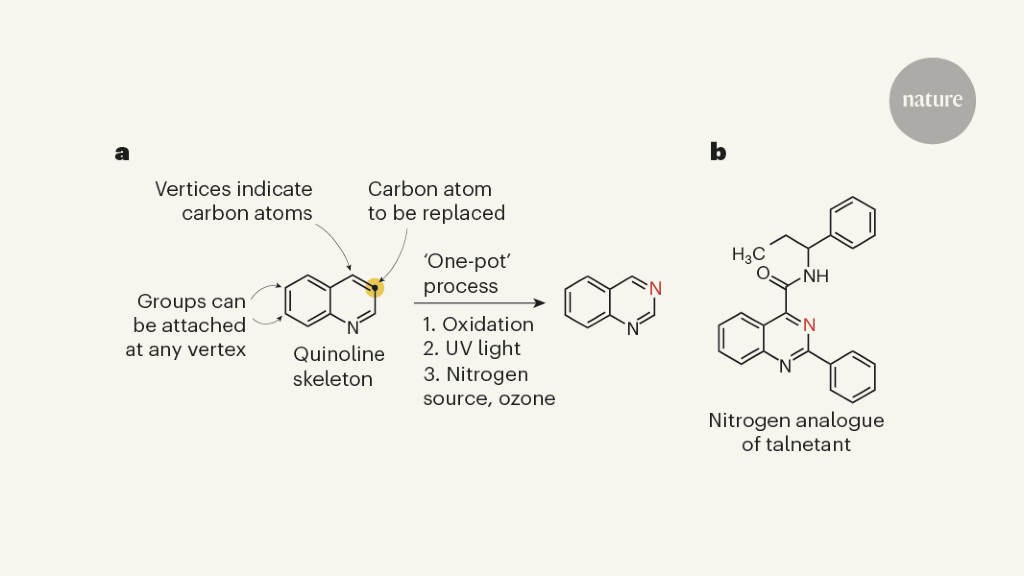
Nitrogen, oxygen and sulfur can be found in the carbon frameworks of the active ingredients in pharmaceuticals and agricultural products. A simple way to create analogues of biologically active compounds would be provided by modifying the skeletons with a different element. However, this conceptually straightforward and potentially versatile transformation is missing from much of the extensive repertoire of reactions used for organic synthesis. In a paper in Nature, Woo et al.1 present a practical strategy for replacing a carbon atom with a nitrogen atom in molecules known as quinolines, which are often found in pharmaceuticals — providing an inspiring example of a single-atom substitution.
Schnherr and Cernak called for a new C–H methylation reaction for drug discovery. The person said agnew. The method of taking Chem. Int. There is an Ed. 52, which was published in 2013).
Pennington, L. D. & Moustakas, D. T. The necessary nitrogen atom: a versatile high-impact design element for multiparameter optimization. J. Med. Chem. 60, 3552–3579 (2017).
The structural diversity, substitution patterns, and Frequency of nitrogen Heterocycles among the U.S. FDA approved pharmaceuticals were analyzed. J. Med. Chem. 57 was published in 2014; it was available on the internet.
The perspective on the chemistry of the drug macitentan. It’s called Bioorg. Med. Chem. The Lett. 26, in particular, has been published in recent times.
In Successful Drug Discovery is a book about successful drug discovery.
It is possible to access diverse oxygen Heterocycles through oxidation of benzylic tertiary alcohols. Org. Lett. 18 of 1896-1999.
Chemical equivalent of arene monooxygenases: a synthesis of arene oxides and oxepines. J. Am. Chem. Soc. 142, 10125–10131 (2020).
Synthesis of alkyl pyridines by nitrogen atom insertion: A group that cut and sewed. J. H., B. R., N. C. Morofuji, K. Mor
Kennedy, S H., Dherange, B. D., Berger, K.J., and M. D. Skeletal editing using nitrogen deletion. The book Nature 593 was published in the year 2011.
The zinc/nickel tandem catalysis is needed to insert Boron into the alkyl ether bonds. Science 372 and 1752 will be published next year.
T. Morofuji, K. and N. Inagawa were involved in the preparation of para-substituted pyridines into meta-substituted anilines. Org. In Lett. 23, 6126–6130, there is a reference to 2021.
Reisenbauer, J. C., Green, O., Franchino, A., Finkelstein, P. & Morandi, B. Late-stage diversification of indole skeletons through nitrogen atom insertion. Science 377, 1104–1109 (2022).
The reactions of azOXY compounds, nitrones and aromatic N-oxides were studied. Chem. Rev. 70, 231–265 (1970).
Chen, P., Billett, B. A., Tsukamoto, T., and Yong, G. are all part of a group that cut and sewed. ACS Catal. 7, 1340–1360 (2017).
De Lescure, L., Patons, R.S., and McNally, A. were involved in the halogenation of 3-position pyridines through Zincke imine intermediates. Science 372, 381, 411, 411.
Source: Carbon-to-nitrogen single-atom transmutation of azaarenes
Citation of Cyclic Acids as Restriction Enzymes and their Use in DNA Cutter: The Case of AMG 232
There is a short history of the restriction enzymes used in the DNA cutter. Nucleic Acids Res. 42, 3–19 (2014).
Willand- Charnley, Fisher, T J., Johnson, B.M. and Dussault, P. H. are interested in Pyridine as an organocatalyst. There is an etymology to the word Org. Lett. 14, 2242–2245 (2012).
C8selective acylation of quinoline N-oxides with – oxocarboxylic acids via regioselective C–H bond activation. They have a organization. Lett. 18, 3722–3825.
P., Hill, M. R., W., Kristufek, S. L., and Johnson all studied clip chemistry. The Chem. Rev. 121 will be published in 2021.
Cochran, B. M. Development of a commercial process to prepare AMG 232 using a green ozonolysis–Pinnick tandem transformation. J. Org. Chem. 84, 4763–4779 (2019).
J. A.Ragan and others were present. There is a safe procedure of large-scale ozonolysis, which involves the preparation of the adduct of 2-hydan-2-carboxaldehyde and its utility in a reductive amination. The process Res. The year 2003 is called the Dev. 7, 155–160
Source: Carbon-to-nitrogen single-atom transmutation of azaarenes
Formamide-Catalyzed Nucleophilic Substitutions and Their Role in Neurokinin Reservoir Transmembranes
Malherbe, P. et al. Me-Talnetant and Osanetant interact within overlapping but not identical binding pockets in the human tachykinin neurokinin 3 receptor transmembrane domains. 73, 1736–1750 was published in 2008.
Dexter, D. L. et al. Activity of a novel 4-quinolinecarboxylic acid, NSC 368390 There are experimental tumors, and they are using a chemical called’methyl-4- quinolinecarboxylic acidsodium salt’. Cancer Res. 45 was published in 1985.
B. J., C. Y., and R.L Ozonolysis of 1, 1-, 2-, and 3-dimethylethene. J. Organization. Chem. 55 was published in 1990.
Gollnick and Koegler discussed the thermal transformation of oxazole endoperoxides. The Lett. 29, 1007–1012, was published in 1988.
The model of Formamide-catalyzed nucleophilic substitutions and their mechanism of action are presented. There was an article in theACS Catal.10 in 2020.

