An egocentric steering signal is converted into an allocentric goal
Effects of neural activity on PFL2 phases in the brain for electrophysiological and electrophysiology experiments. I. The case of PBac flies
MCFO, w[1118] P{y[+t7.7] w[+mC]=R57C10-FLPG5}su(Hw)attP8; PBac{y[+mDint2] w[+mC]=10xUAS(FRT.stop)myr::smGdP-HA}VK00005 P{y[+t7.7] w[+mC]=10xUAS(FRT.stop)myr::smGdP-V5-THS-10xUAS(FRT.stop)myr::smGdP-FLAG}su(Hw)attP1.
Flies used for the experiments were isolated the day before the experiment so they could not be used in other experiments. Female flies were used for the calcium experiments. For electrophysiology experiments, including the iontophoresis experiments, we used female flies 16–30 h posteclosion. No circadian restriction was imposed for the time of experiments.
To label PFL3 cells only for calcium images. 9i and 10a) we used +; 57C10-AD/UAS-tdTomato; VT037220-DBD/UAS-GCaMP7f.
Our model contains 1,000 PFL2 units, 1,000 PFL3R units, 1,000 PFL3L units and 1,000 goal cell units. We chose to use a large number of units for these cell types, so that model output resembles a quasi-continuous function over neural space, because this makes it easier to see how spatial patterns of ensemble neural activity might resemble a sinusoidal function. In reality there are only 12 PFL2 cells, 12 PFL3L cells and 12 PFL3R cells in the brain. We have verified that matching the numbers with neural activity does not alter our conclusions.
We calculated the relative changes in PFL2 bump phase and head direction in 1.5 s bins as shown in Fig. 2d. In each time window, we took the difference between start and end points for θ or bump phase. Positive differences represent a clockwise shift while a negative difference represents a counterclockwise shift. The relationship between changes in and changes in bump phase was the strongest when there was a 200 ms lag. The line of best fit for the relationship between the two variables was found with the polyfit and polyval functions. The correlation coefficients and P value of the relationship were calculated using the corrcoef function. We excluded indices where the adjusted r2 value of the sinusoidal fit for bump parameters was below 0.1 or the fly was not moving.
If the goal input is not influenced by the right–left difference in PFL3 activity, the activation function must be non-linear. We use S as our default, except in figs. 5c,d and 5j,k, where we investigate the effect of lowering S.
,f(Stimes (theta – theta )
Neural representation of the goal direction as a sinusoidal pattern and its implication for steering signals in the hemibrain connectome
We model the neural representation of the goal direction (θg) as another sinusoidal pattern over neural space, which is reasonable, because the goal direction can be thought of as just a special head direction, and head direction is represented as a sinusoid. We assume the goal input is the same in PFL2 and PFL3R as it is in PFL3L. The goal representation will shift if the offset of the head direction system is changed. As the goal direction rotates rightward (clockwise), the peak of activity in goal cells will move leftward across the fan-shaped body. We use A = 1 in our model implementations, so that the amplitude of the goal signal is equal to the amplitude of the head direction signal, but some of our results can be potentially explained by a mechanism that modulates A (Extended Data Fig. 8).
We get an expression that predicts steering as a function of PFL2 and PFL3 activity by combining equations. The DNa03 output is thought to be in the right and left hemispheres. The weight array is written using the abbreviations D2 (DNa05), D3 (DNa03) and P2 (PFL2).
It is a starting point for the rmldtpropto rmDNa03.
The model is intended to understand how steering signals arise from the head direction system. The steering signal is the right– left difference in the activity of DNa03 descending neurons and has been shown to influence steering.
Analysis of the data obtained from the hemibrain connectome was done using a software package named neuprintr natverse.
There are 20 complete PFL2 cells, 19 complete PFL3 cells and one nearly complete DNa03 cell in the hemibrain connectome. Although the axon terminal of DNa03 is not present in the hemibrain dataset, DNa03 makes many output synapses in the brain, so there are still many EM images of the presynaptic sites within this cell. A recent computation, called 50,51 automatically infers transmitter identification from the hemibrain dataset and predicts which of the 12 PFL2, 13 PFL3 and 1 DNa03 cells are cholinergic. The system predicts transmitters on a per-Synapse basis, with an error rate that varies with cell and transmitter type The majority of pre-synapses are predicted as cholinergic, while the second most commonly predicted transmitter is glutamate. 84.9% of high-confidence presynapses are predicted cholinergic; the second most commonly predicted neurotransmitter is glutamate. The model was train using 3,094 hemibrain neurons in its ground-truth data, which were identified as cholinergic using light microscopy and antibody staining. Among this ground-truth population, 73% of presynapses are correctly predicted as cholinergic. The predictions are available from the ref. 51.
In Extended Data Fig. 4e, to compute the stimulation location angle, we treated the fraction of pixels of each column ROI that were inside the stimulation ROI (see red colour map in Fig. As an array 2c,d. The stimulation location angle is computed using the same population average method as in the FC2 phase. We then took the mean difference between the two stimulation phases (A and B) for each fly. To compute the mean FC2 phase position during the stimulation period (Extended Data Fig. We decided not to include timepoints when the fly was standing still.
We first used a bandpass filter to make sure we weren’t detecting spikes. We then detected peaks in the filtered Vm trace above a specified threshold, spaced by >5 ms, using the SciPy function signal.find_peaks. Although this criterion means we could not detect spike rates above 200 Hz, the activity levels of all our cells stayed well below this upper limit. Different cut-off frequencies and thresholds were hand selected for each cell so as to yield spike times that matched what one would expect from visual inspection of the data. To remove spikes from the Vm trace. 3c,d and Extended Data Figs. 6d, 7c,d and 9b,c—we discarded Vm samples within 10 ms of a spike by converting those samples to empty entries (that is, the not-a-number (NaN) data type).
The 5s before or after the cue jump were used for the calculated IPSP frequencies. The change in frequency before jump versus after jump was then compared to change in head direction relative to the cell’s preferred head direction produced by the cue jump. This was determined by first finding the absolute angular difference between the head direction before the jump and the cell’s preferred head direction (see section ‘Computing preferred head direction’) and doing the same for the new head direction following the cue jump. Then the precue jump value was subtracted from the postcue jump value. The negative value indicates that the head direction was less direct to the cell’s preferred head direction after the jump, while the positive value shows that the distance between head direction and cell’s preferred head direction increased. The change in IPSP frequency was then plotted against the change in the distance from the cell’s preferred head direction for each jump. The polyval and polyfit functions were utilized to find the best fit for the relationship between the two variables. The significance of the relationship between change in frequency and change in direction compared to cell identity was determined using an unbalanced two-factor ANOVA.
The plots shown in their entirety. 2a,b and Extended Data Fig. 4, data from the ±10 s period around each ATP pulse were averaged within individual flies to get the fly-averaged response to the 100 ms, 200 ms, 300 ms and 500 ms pulses for the membrane potential, forward velocity, sideways velocity and rotational velocity (each condition had at least four repetitions per fly). We calculated the grand mean and the s.e.m. across all flies using these per-fly averages.
To track the amplitude and phase of PFL2 activity for analyses in Figs. 2c–g and 5j,k and Extended Data Figs. 3, 5 and 8, a sinusoid was fit independently to each time point of the z-scored ΔF/F activity across fan-shaped body and protocerebral bridge imaging trials:
The analysis for the head direction tuning is shown in the picture. We used a threshold of 0.7 and only used the data from segments with 0.7. We lowered the threshold on ρ for this analysis because we needed to include a larger number of time points in the analysis, to improve the resolution for binning the activity of cells into groups defined by θp − θg.
The values chosen for parameters ε and L were conservative, in that they tended to break up portions of the fly’s trajectory where one might have considered the fly’s goal to have remained unchanged into smaller bouts. We preferred this bias over the risk of potentially lumping two bouts together, where the fly’s true goal angles might have been different.
Rh is the ratio between the left and the right.
Online ball-tracking imaging of the spherical treadmill for calcium and MRI using a machine vision software system, Fictrac V.2.1
The end of each stack on the same volumetric acquisition card was indicated by a signal from the software we use to perform calcium scans. These imaging time points were then resampled to the ball-kinematic data update rate of 60 Hz, allowing us to align the acquired volumes. Ball tracking data was reanalyze to match the sampling rate of the electrophysiology data, but alignment wasn’t required because the electrophysiology data was collected on the same card.
The position of the spherical treadmill was computed online using machine vision software (Fictrac v.2.1) and output as a voltage signal for acquisition. The signal was converted into radians for analysis. Signals were then low-pass filtered using a second-order Butterworth filter with 0.003 corner frequency and downsampled to half the ball-tracking update rate. The equation was calculated using a function. Artefactually large velocity values (greater than 20 rad s−1) were set to 20 rad s−1, and timeseries were then smoothed using the smooth function in MATLAB (using the loess method with an 33 ms window) and resampled to 60 Hz, the ball-tracking update rate. Forward and sideways velocities were then converted to millimetres per second while yaw (rotational) velocity was converted to degrees per second.
$${y}{i}=\frac{{x}{i}-\,\underline{X}}{{\rm{MAD}}},{\rm{where}}\,\underline{X}={\rm{median}}\,{\rm{of}}\,X,\,{\rm{MAD}}={\rm{median}}\,(| {x}_{i}-\underline{X}| )$$
Precision determination of the modified Z-Score of flies that expressed GCaMP under the PFL2 and PFL3 lines at z-stacks
Analysis was performed in either MATLAB 2019 or MATLAB R2021a. The data contained 33 flies that expressed GCaMP under the control of the PFL2 line and 23 flies that expressed GCaMP under the PFL3 line. The motion correction was done on the x, y and Z axes. Each region of interest (ROI) was defined across the z-stack. For each ROI ΔF/F was calculated with the baseline fluorescence (F) defined as the mean of the bottom 10% of fluorescence values in a given trial (600 s in length). The modified Z-Score was calculated from this measurement using the median absolute deviation, we refer to as the z-scored F/F.
The primary antibodies contained mouse anti-Bruchpilot and rat anti-Flag. NBP1-06712B) and rabbit anti-HA (1:300, Cell Signaling Technology, catalogue no. 3724S). The secondary solution contained a 487 goat anti-rabbit. The goat anti-rat was catalogue no. A-1109) BothAlexa Fluor and 612-156-120. goat anti-mouse (1:500, Invitrogen, catalogue no. A30534. The tertiary antibody solution contained DyLight 550 mouse anti-V5 (1:500, Bio-Rad, catalogue no. The name is MCA 1372D450GA.
The brains of flies were fixed in 4% paraformaldehyde after being torn from the flies 1–3 days posteclosion. Brains were washed with PBS before adding a blocking solution containing 5% normal goat serum (Sigma-Aldrich, catalogue no. G9023 is also referred to as G902. In PBS, there is 0.44% of Triton-X. for 20 min. Brains were then washed in PBS and put into a secondary antibody with a blocking solution for about 24 hours at a room temperature. Primary and secondary antibodies were protocol-specific (see below). Brains were placed in an antifade mounting medium after being washed with PBS. Before mounting, a tertiary incubation step for about 24 h at room temperature was performed. Mounted brains were imaged on a confocal microscope using a 40, 1.15 NA oil-immersion objective. Image stacks comprised 50 to 200 z-slices at a depth of 1 μm per slice. There was a resolution of 1,024. For visualizing Gal4 expression patterns, the primary antibody solution contained chicken anti-GFP (1:1,000, Abcam, catalogue no. ab13970) and mouse anti-Bruchpilot (1:30, Developmental Studies Hybridoma Bank, nc82). The goat anti-chicken is contained in the secondary antibodies solution. A11039) and Alexa Fluor 633 The goat is anti-mouse. A 2050. For visualization of cell fills after patch-clamp recordings. Alexa Fluor 568 is a monograph of the drug streptavidin. S11226 was added to the secondary solutions.
We illuminated the fly’s brain via an 850 nm LED (Thorlabs) coupled to an achromatic lens pair (MAP10100100-A, Thorlabs) that focused the light from the LED onto a small spot on the fly’s head. They used borosilicate patch pipettes that had resistances of 6-13 M. There was zero injected current when recording and the recordings were conducted in current-clamp mode. The voltage signal was low-pass filtered at 4 kHz before sampling at 10 kHz. Plots have been changed to accommodate a 13-mV liquid-liquid junction potential. We visualized the recorded cell by using a manual Z-stack on our epifluorescence patch-clamp microscope and illuminating with a 565-ms-illuminating light. We stained the brain to confirm the identity of the patched cell.
We used a circular panorama built from modular square panels to show visual stimuli. The arenas had twelve panels in total, with two panels tall. The upper panel behind the fly was removed to make room for the camera view and light source. In all experiments, the modular panels contained blue LEDs with peak blue (470 nm) emission; blue LEDs were chosen to reduce overlap with the GCaMP emission spectrum. For calcium imaging experiments, four layers of gel filters were added in front of the LED arena (Rosco, R381) to further reduce overlap in spectra. For electrophysiology experiments, only two layers of gel filters were used. We put on a final layer of a diffuser to prevent reflections in both cases. The visual cue was a bright (positive contrast) 2-pixel-wide (7.5°) vertical bar. The bar’s height was the full two-panel height of the area, except for a single visual display panel, where the bar was half this height. The bar intensity was set at a luminance value of 4 with a background luminance of 0 (maximum value 15).
Experiments used an air-cushioned spherical treadmill and machine-vision system to track the intended movement of the animal. The treadmill was made from foam and sitting in its holder were three-dimensionally printed. The ball was floated with medical-grade breathing air (Med-Tech) through a tapered hole at the base of the holder using a flow meter (Cole Parmer). For machine-vision tracking, the ball was painted with a high-contrast black pattern using a black acrylic pen and illuminated with an IR LED (880 nm for two-photon experiments; M880L3, Thorlabs, or 780 nm for electrophysiology experiments; M780L3, Thorlabs). Ball movement was captured online at 60 Hz using a CMOS camera (CM3-U3-13Y3M-CS for two-photon imaging, or CM3-U3-13Y3C-CS for electrophysiology, Teledyne FLIR) fitted with a macro zoom lens InfiniStix (68 mm ×0.66 for two-photon, InfiniStix 94 mm ×0.5 for electrophysiology). The camera faced the ball from behind the fly (at 180°). Machine vision software (FicTrac v.2.1) was used to track the position of the ball43 in real time. The forward axis ball displacement, yaw axis ball displacement, forward ball displacement and gain-modified yaw ball displacement were recorded along with other experimental time series data using a custom Python script. The gain-modified yaw ball displacement voltage signal was also used to update the azimuthal position of the visual cues displayed by the visual panorama.
Microscopy and Control of the Split-Gal4 Line for Protocerebral Bridge, Fan-Shaped Body, and LAL
The patch pipettes were pulled from the capillary glass with a horizontal pipetter to a resistance range of 9–13. The pipettes contained an internal solution consisting of 140 mM KOH, 140 mM aspartic acid, 1 mMKCl, 10 mM Hepes, 1 mMEGTA, and 4 mM MgATP. The filters for Na3GTP and 15 mM Neurobiotin is 0.22 m.
We used a two- photon microscope as well as a galvo-galvo-resonant Scanhead, which had a 1.10 NA objective. For volumetric imaging, we used a fast piezoelectric objective scanner (Thorlabs PFM450E). We used the wavelength-tunable laser with dispersion compensation to excite GCaMP. In order to control microscopes, all image acquisition and control was done using the ScanImage 2021, Premium with vDAQ hardware and custom MATLAB script. The region for image of the fan-shaped body was 150 250 pixels, while the region for image of the LAL was 150 400 pixels. Each volume received a 10–12 slice in the z axis, which resulted in a 6–8 Hz volumetric scanning rate. For experiments using the PFL2 splitGal4 line, we imaged in the Protocerebral bridge, fan-shaped body or LAL. For experiments imaging the mixed PFL2 and PFL3 split-Gal4 line, we only imaged in the LAL.
The following stocks were obtained from WellGenetics: w[1118];P{VT007338-p65ADZp}attP40/CyO;+ (SWG9178/A), w[1118];P{VT033284-p65AD}attP40/CyO;+ (A/SWG8077). We constructed a split-Gal4 line with the expression in the LAL being specific to PFL2 and PFL3 cells. We used the multi-color flip out to visualize the line’s single-cell morphologies and also the anti-GFP staining to determine its expression. The brain expression line is non-specific, but specific for PFL2 and PFL3 in the LAL. We also constructed a split-Gal4 line to target PFL2 neurons, +;P{VT033284-p65AD}attP40; P{y[+t7.7];P{VT007338-Gal4DBD}attP2. MCFO was used to visualize single-cell morphologies after we validation the expression of this line. The line shows expression in various peripheral nerves, but only in certain PFL2 cells within the central complex.
We obtained the following stocks from the Bloomington Drosophila Stock Center (BDSC), the Janelia FlyLight Split-Gal4 Driver Collection or from other laboratories: VT000454-p65AD; VT001980-GAL4.DBD (SS02239)51, VT000355-p65AD (attP40)51, 57C10-p65AD (attP40) (BDSC 70746), VT037220-Gal4.DBD (attP2) (BDSC 72714), R60D05-Gal4 (attP2) (BDSC 39247), empty-AD; empty-DBD (BDSC 79603), 27E08-p65AD (BDSC 70048), UAS-2xeGFP (Dickinson laboratory), 20XUAS-IVS-jGCaMP7f (VK05) (BDSC 79031), 20XUAS-IVS-jGCaMP7f (su(Hw)attP5) (BDSC 80906), 10XUAS-sytGCaMP7f (attP2) (BDSC 94619), UAS-tdTomato (attP40) (BDSC 32222), UAS-CsChrimson-tdTomato (VK22) and UAS-CsChrimson-tdTomato (VK05) (gifts from D. Anderson, B. Pfeiffer and G. Rubin), UAS-mCD8-GFP (attP2) (BDSC 32194), UAS-RedStinger (attP40) (BDSC 8546), hs-FLPG5.PEST (BDSC 64085), pJFRC99-20XUAS-IVS-Syn21-Shibire-ts1-p10 (VK00005) (gift from G. Rubin), UAS-TNT(E) (BDSC 28837) and UAS-TNT(Q) (BDSC 28839).
Drosophila melanogaster flies were raised at 25 °C on a 12-h light:dark cycle. All physiological and behavioural experiments were performed on 1- to 4-day-old female flies. For optogenetic experiments, experimental and control crosses were kept in a box with a blue gel filter (Tokyo Blue, Rosco) as a cover—to minimize exposure to light within the excitation spectrum of CsChrimson while also not keeping the flies in complete darkness; eclosed flies from such experiments were placed onto food containing 400 µM all-trans retinal for at least one day.
Crossing Lines Towards UAS-Red Stinger: Converting an Allocentric Goal into an Egocentric Steering Signal
We used +/; +/+; U AS-GCaMP7f60D05-Gal4 or +, and UAS-tdTomato.
To discover the expression pattern of VT029306-DBD. 1a,b), 57C10-AD; VT037220-DBD (Extended Data Fig. VT00455-AD; VT03720-DBD are included in the 1d,e. 1f,g) and 27E08-AD; VT037220-DBD (Extended Data Fig. We went across each of these lines to UAS-RedStinger.
Source: Converting an allocentric goal into an egocentric steering signal
Experimental evaluation of the ethological origin of wind-induced memory of fly activity in an allocentric direction for upwind running airflow
Each fly was able to experience six different wind directions for an entire eighteen trials of the wind-induced memory task. The 6 wind directions we presented were –135°, –90°, –45°, +45°, +90° and +135°. These angles were selected based on two considerations. First, we wished to avoid allocentric wind directions in which the bar would be located in the 90° gap at the back of the LED arena when the fly is oriented upwind (that is, a 180° allocentric wind direction) since without a visual cue flies are expected to have a poorer estimate of their heading angle. If we were to orient toward a bar in an allocentric wind direction, the bar would be located directly in front of the fly. Wind directions were presented in one of two orders, either (–135°, –90°, –45°, +45°, +90°, +135°) or (+135°, +90°, +45°, –45°, –90°, –135°), with the exact order chosen randomly for each fly. For each trial, airflow remained on for 30 s and was followed by a 2-s, 180° bar jump after the airflow was turned off. The bar jump ensured that if flies simply kept walking straight after the airflow turned off, this would not lead to a high performance index or indication of angular memory. The inter-trial period, which also included the ‘test’ period where we assessed the flies’ wind-induced heading memory, lasted 60 s. There was a 3-min period in between the end the wind period of the last trial of a wind-direction block and the start of the wind period of the next wind-direction block. We collected one recording file per fly. In preliminary experiments, it seemed that flies formed stronger wind-induced memories of an allocentric direction when the six possible wind directions were presented in a consistent, clockwise or anticlockwise sequence—as was done in the reported experiments—rather than appearing in a completely random sequence. This observation makes ethological sense in that allocentric wind presented from very different directions over time might lead flies to downgrade the relevance of wind, very generally, as a useful stimulus for allocentric navigation.
We dissected the brains and incubated them in either 2% paraformaldehyde (PFA) for 55 min at room temperature or in 1% PFA overnight at 4 °C. We blocked and de-gassed brains in a blocking solution consisting of 5% normal goat serum (NGS) in 0.5% Triton X-100, phosphate buffered saline (PBT).
For GFP and RedStinger labelling experiments (Extended Data Fig. We used a primary solution of 1:100 chicken anti-GFP (Rockland, 600-901-215), 1:500 rabbit anti-dsRed (TAkara 622496) and 1:10 mouse anti-Bruchpilot. For it’s extended data figs. 11i,j) we used a primary solution of 1:1,000 rabbit anti-TNT (Cedarlane, 65873(SS)) and a secondary solution of 1:800 goat anti-rabbit:AlexaFluor 488 (Invitrogen A11034).
For visualizing biocytin-labelled neurons after patch-clamp experiments (Extended Data Fig. 6a), the primary antibody solution we used was 1:10 mouse anti-nc82 in 1% NGS/PBT and the secondary antibody solution was 1:800 goat anti-mouse:Alexa Fluor 488 and 1:1,000 streptavidin:Alexa Fluor 568 (Invitrogen S11226) in 5% NGS/PBT.
Brains were mounted in Vectashield and images were acquired using a Zeiss LSM780 confocal microscope with a 40×/1.20 NA water-immersion objective or a 10× air objective.
Source: Converting an allocentric goal into an egocentric steering signal
Temperature control of plate-tethered flies on a saline/agarose gel for closed-loop wind experiments
For closed-loop wind experiments, in which physiology was not performed simultaneously, we pin-tethered flies to a tungsten pin. The glue was put between the head and the thorax. The wings were fastened to the thorax with glue around the scutellum.
When food is not eaten for 8–16h and heated to 34C, wild-type flies typically perform menotaxis. In the present study, we noticed that for some genotypes, the same level of food deprivation would yield unhealthy flies. As such, we opted for a shorter period of food deprivation for most experiments. We typically do 3 h of experiments after tethering flies. During this interval, we kept tethered flies inside a box with a wet piece of tissue paper to prevent desiccation. Before tethering flies on agarose, we placed them on plain agarose. In all plate-tethered experiments, we heated the tethered fly by perfusing 26–30 °C saline over the fly’s head using a closed-loop temperature control system (Warner Instruments, CL-100). We heated flies using a 980nm lasers called RDHS980-200-3. The intensity of the laser was controlled via pulse-width modulation in closed loop with a temperature reading from a thermal camera image (C2, Teledyne FLIR). The temperature set point was assigned to be 32 C for both experiments.
Source: Converting an allocentric goal into an egocentric steering signal
Allocentric Wind Direction Calculation Using the Bar and Spigot Difference in Extended Data Fig. 6a, 6c and 6c
In Fig. 6, for each trial, the allocentric wind direction was computed by taking the mean of the difference between the bar position and the spigot angle, at every timepoint, during the time period when the airflow was on. This value was not comparable to the allocentric wind direction set by our code due to latencies associated with the air delivery spigot having to physically move to deliver air from a new direction. The set point and wind direction could be different.
For the fits shown in Extended Data Fig. 6d, the data were fit to (A\cos (H-{H}{\text{pref}})+{V}{0}), with A, Hpref and V0 as fitting parameters, by minimizing the squared difference between this expression and the data points.
For Extended Data Fig. 4c, an ROI was considered inside the stimulation ROI if it had at least one pixel within the boundaries of the stimulation ROI scan path and was otherwise considered outside the stimulation ROI. In Extended Data Fig. ROIs that weren’t inside the stimulation area were analysed. The change in the column ROI ΔF/F0 was computed by dividing the mean ΔF/F0 during the 30 s stimulation period by the mean the ΔF/F0 during the 5 s before the stimulation. To calculate an ROI’s distance from the stimulation site (in number of ROIs), we first defined the stimulation site as the column ROI with the highest fraction of pixels inside the stimulation ROI. For each ROI we then computed its wrapped distance in number of ROIs. The distance between column two and column 15 is wrapped up by the number of columns in our analysis. In the extended data fig., we could see that our stimulation ROI could overlap with other columns. There aren’t column ROIs with a distance of one.
In Extended Data Fig. The peaks were found in the boxcar filter by using theSciPy65 function signal. In addition, we required that the FC2 PVA within 1 s from the peak phase velocity was above 0.15 at all timepoints and that the mean PVA during this time was above 0.25. These criteria helped ensure that the genuine changes in the FC2 bump position were detected rather than the spurious changes caused by a poorly estimated phase. To overlay all of the detected changes in FC2 phase position, as well as the flies’ heading during these moments (Extended Data Fig. 3g), we aligned traces to the start of the peak in phase velocity. In order to combine traces where the peak in the FC2 phase velocity was either positive or negative, we flipped the FC2 phase for traces where the peak phase velocity was positive.
The FC2 phase can be found in the fan shaped body. The calculation was done by us based on the phase of the bridge described previously. We used the coefficients of length 16 to figure out the glomeruli F/F0 in the bridge. The phase of the Fourier spectrum at a period of 8.5 glomeruli was used as the EPG phase.
The CaIMAn64 python package was used to register two- photon images for motion artefacts. The left side of the LAL and the columns of the bridge were defined using a custom graphical user interface written in Python. The local correlation image and time average signal were used to draw the ROIs. We used a semi-automated method to define columns in the fan-shaped body. We first defined a return on investment for the entire fan-shaped body. This ROI was then subdivided into 16 columns of equal angular size using two lines that defined the lateral edges of the fan-shaped body. For each ROI, we defined ΔF/F0 as equal to (F − F0)/F0, where F is the mean pixel value of an ROI at a single timeframe and F0 is the mean of the lowest 5% of F values.
The yaw, pitch, and roll angles of the ball were recorded at 50 Hz with the ball camera, and then aligned to our data files. The data was shifted backward in time by 30 ms due to the measured pulse speed between the triggering pulse and finishing the image processing. For closed-loop wind experiments which didn’t need to align data, no camera triggered signals or samples were used.
All time series data was converted to a digital format with the use of the PClamp software suite, except for two- photon images which were saved as tiff files. To align imaging data with behavioural data, we used a voltage signal of the y galvo flyback, which marks the end of an imaging frame, as an alignment point. For each imaging volume, the midpoint between the start of the volume’s first z-slice and the end of its last z-slice was used as its time stamp.
Source: Converting an allocentric goal into an egocentric steering signal
Mapping the Bar Jump in Central Complex 41,42 by Using a Velocity Relative to the EPG Heading Estimate
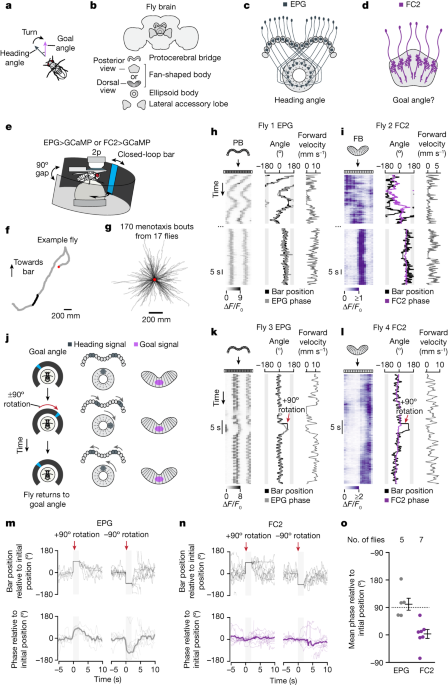
In all physiological experiments, we allowed the fly to walk in closed loop with the bar for approximately 5–30 min as we prepared for data collection (that is, during desheathing and seal attempts in patch-clamp measurements or during ROI selection in imaging experiments). This time period gave the fly experience with all possible angular bar positions, which is expected to reinforce the formation of a stable map between the position of the bar on the screen and the EPG heading-estimate in the central complex41,42.
The FC2 phase can be assessed relative to its position immediately prior to the bar jump by performing a V- test with a known mean angle. The V-test we performed was to see if the bar is tracked during a bar jump. The same tests were applied to it. 3e yielded μ = 0° (P = 7.69 × 10−8) for the FC2 phase and μ = 90° (P = 2.49 × 10−5) for the EPG phase.
In Fig. 6f and Extended Data Fig. 11b,c, the absolute distance to wind was taken to be absolute value of the flies’ heading relative to the wind direction, computed as described above.
Source: Converting an allocentric goal into an egocentric steering signal
Patch-clamp imaging with a supercold xy plane and a pi:Sapphire laser pulsed photon imager
We performed patch-clamp experiments as described previously33, with some changes indicated below. We gave the brain an extracellular solution which bubbled with oxygen and 5% CO2. The mM contains a mixture of 103 NaCl, 3 KCl, 5TES and 10 trehalose dihydrate. 140 aspartates, 1 KCl, 10 HePES, 1EGTA,0.4 Na3GTP, 4 MgATP were part of the solution61 in mM. Some of the solutions included was 13m biocytin hydrazide and 20 mM. Alexa Fluor 568 (Invitrogen, A10437), which could be used to fill the neuron for subsequent verification of the identity of the cell from which we were recording.
We performed a two- photon calciumography as described. We used a Scientifica Hyperscope and a Chameleon Ultra II Ti:Sapphire femtosecond pulsed laser (Coherent) tuned to 925 nm. We performed volumetric imaging, using galvo-galvo mode (Cambridge Technologies MicroMax) to scan the xy plane and a piezo device (PI, P-725.4CA) to move a 16×/0.8 NA objective (Nikon) along the z axis. The emission light was split by using a dichroic mirror. We used a 500-550 nm bandpass filter for the green signal and a 590–650 nm bandpass filter for the red signal. Emission photons were detected and amplified using GaAsP detectors (Hamamatsu, H10770PA-40). ScanImage60 (2018b) software was used to control the microscope.
For Fig. 5a–d, we used ScanImage’s MultipleROI feature to define two 50 × 50-pixel ROIs for each side of the LAL. The volume rate was 9.16 mph, and we scanned the LAL with two slices of paper. The image was scanned using a 128 64-dpiROI with 3 Z slices. The laser power we used was measured after the objective. Imaging recordings lasted up to 26 min. Over time, the fly’s brain would slowly sink over a recording. To correct for this motion, we manually adjusted the position of the objective via a microscope-stage motor during the recording.
For Fig. 5e–h, we alternated from stimulating the left or right LAL. The stimulation ROI was relocated to the LAL where there was no CsChrimson-tdTomato expression. These experiments used a stimulation power of 70 mW. The LAL was scanned with a singlez-slice which had an acquisition rate of 4.97Hz and a duty cycle of 0.23.

