There are many abortion patients who have no other choice but to use an ulcer drug
Medication abortion is still banned or restricted by the FDA after the Supreme Court ruling on the 1873 Comstock Act in the U.S.
The FDA’s pharmacy certification for mifepristone requires pharmacies to track shipments and to keep records of prescribers, recipients and lot numbers of each drug dispensed. This “inhibits the creation of a secondary distribution network for this drug,” Grossman said, such as if people in a state with access send the drug to those in abortion-restriction states.
“It’s not at all clear that many or all or most pharmacies, or pharmacies in more rural areas, or pharmacies in red states will do so in ways that meaningfully increases access to medication abortion,” Leah Litman, a professor of law at the University of Michigan, said in an email Wednesday.
Laws vary by state, but the medications can be taken up to 11 weeks after the first day of the last menstrual period. Depending on where you live, a person can go to a state that is legal for abortion to get the pills or they could use a telehealth prescription.
Medication abortion is used in more than half of abortions in the US, outpacing surgical procedures for the first time in 2020, according to the Guttmacher Institute, a research group that supports abortion rights.
The Office of Legal Counsel opinion dated December 23, 2022, said that the 1873 Comstock Act “does not prohibit the mailing of certain drugs that can be used to perform abortions where the sender lacks the intent that the recipient of the drugs will use them unlawfully.”
In the wake of the Supreme Court ruling, Attorney General Merrick Garland issued a statement promising to work with the FDA and other federal agencies to protect access to such drugs, which some states have sought to ban.
The change partially implemented last year, as the Biden administration decided to no longer enforce the requirement that women pick up the medicine in person. The action on Tuesday formally updates the drug’s labeling to allow more retail pharmacy to sell the pills if they complete a certification process.
abortion is still banned or restricted in places that it was before the FDA update, said Elizabeth Nash, a principal policy associate of state issues at the Guttmacher Institute.
Two drugmakers that make brand-name and generic versions of abortion pills requested the latest FDA label update. Agency rules require a company to file an application before modifying dispensing restrictions on drugs.
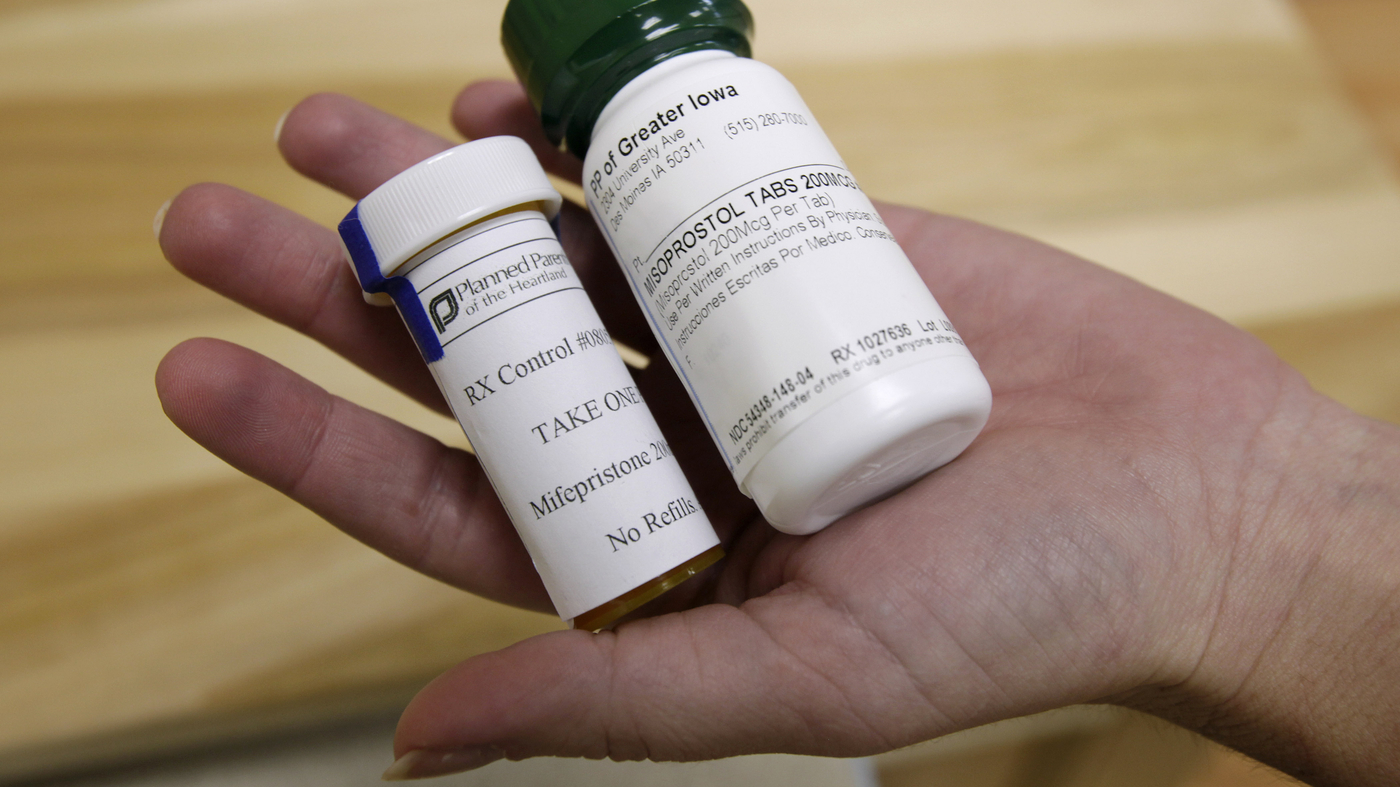
In a medication abortion, mifepristone is used with another drug called misoprostol to end a pregnancy. Mifepristone blocks a reproductive hormones that is needed for a fetus to grow. The drug can be distributed by the pharmacy.
There are many side effects, but bleeding is a common one. More than four million US women have used the drug since it was approved by the FDA.
Several FDA-mandated safety requirements remain in effect, including training requirements to certify that prescribers can provide emergency care in the case of excessive bleeding. Pharmacies that dispense the pills also need a certification.
It’s not clear which other pharmacies will seek certification or what impact it will have on abortion access in places where it’s banned or restricted.
In the states where Republican officials have threatened to take legal action, Walgreens won’t distribute abortion pills. The pharmacy chain said in a statement to NPR on Friday that it’s still taking steps to sell the drug in “jurisdictions where it is legal and operationally feasible.”
The first pharmacy certified to distribute medication abortion was posted on its verified Facebook page Tuesday by Honeybee Health.
But for many pharmacies, it will take some time to review and decide whether to undergo the certification process – and the certification process may be complicated.
Seeing a doctor in person: The impact of the FDA’s more lax abortion laws on patients’ access to healthcare and medication abortion
States can enforce restrictive abortion laws against people who provide, facilitate access to, or obtain medication abortion, but under certain circumstances, the laws cannot be enforced because they undermine the purpose or objectives of federal law.
We do not think anyone should have to go in that way, and as this moves forward, we know there will be a lot of smart lawyers looking at the issue of how they will be incorporated into drugstores and pharmacy chains.
He asked if a state that was prosecuting someone for diversion would have access to those records. If they do that it’s a deterrent to give it to people in states that are banning it.
Many people have difficulty getting healthcare, because of things like transportation or the fact that they have less time off, but this will open doors for them and give them access to care.
She added that the FDA’s conclusion that patients are not at an increased risk by not seeing a doctor in-person, might pave the way for providers who may have previously been nervous about seeking certification.
Sue Liebel, director of state affairs for the Susan B. Anthony Pro-life America, an organization that calls itself “the political arm of the pro-life movement, called the more lax rules “a disaster waiting to happen.”
It is reckless for the FDA to require an in-person exam for these safety regulations. Liebel told NPR that women’s health and safety was not taken into account.
She suggested that as a result, more women “will wind up in the emergency room.” She was not able to point to recent studies that might show an increase in ER visits due to the FDA temporarily lifting the in-person mandate.
“This next policy session will be very interesting, I think,” she said. We’re in new territory here with the decision. t’s going to be a mixed bag to be honest with you in terms of what states will try do and see what works.
Getting the Misoprostol-Alone Protocol at Carafem: How Patients Will Look and Feel at an Abortion Clinic
There’s no data that suggests that people go in waves to seek emergency care because they are really safe and there is a good track record of that.
She thinks clinical care is going to stay the same. “Patients will still be evaluated by experienced clinician. They’ll go through counseling. They’ll be able to talk about whether or not this is the best choice for them.”
The only difference, moving forward she explained, is that in the most dire situations they will no longer have to drive long distances for an exam or wait days to obtain the vital medication. “They’re just going to have much more easy access to it.”
The Trust Women clinic has been in crisis mode for months. The clinic director says the staff is bracing for another waves of uncertainty.
If the judge overturns the approval of that protocol, Brink says the Wichita clinic will only be able to offer patients either a surgical abortion or misoprostol alone. Preparing the staff with extra training on how to communicate with patients about the change is what is needed right now.
“Because it’s different, we’re having to make sure everyone knows what’s going on and how to respond,” Brink says.
A University of California, San Francisco professor is researching a method for avoiding side effects of the oral contraceptives that use prostaglandins.
Leah Coplon, director of clinical operations for Abortion on Demand, a telehealth medication abortion clinic serving 23 states, says her organization is preparing to make the shift to misoprostol-alone if necessary.
They’re coming for something other than mifepristone. Perritt says it will not end with one medication. All of this is at risk.
Carafem began offering the single-drug protocol in 2020 in order to keep abortion accessible in spite of growing threats to abortion access.
Depending on where you are, patients at Carafem can usually get the two-drug protocol for less than $175. In addition to being less expensive than a two-drug protocol, Grant says that about 10% to 15% of her clients choose to use Misoprostol alone.
The next step in ensuring abortion is completely accessible and the FDA won’t approve mifepristone or misoprostol
Grant says that they would have one less option. The next step in making abortion completely accessible is this.
Meanwhile, Elisa Wells, co-founder of the group Plan C Pills, which provides information for people seeking abortion medications online, says her organization will continue to point clients toward alternative sources of both mifepristone and misoprostol.
According to Wells, at least 30,000 people have obtained abortion pills through these networks.
When it’s prescribed off-label, it puts the doctor in a tenuous position because it hasn’t been approved by the FDA for that purpose.
But Blake’s colleague, Alliance Defending Freedom attorney Erik Baptist, says he believes doctors who prescribe misoprostol for abortion could open themselves up to lawsuits.
The current lawsuit doesn’t directly target off-label uses. It’s already used off-label for procedures like abortion and IUDs, and perritt believes it will continue to be used. But she worries about an increasingly murky legal landscape surrounding abortion pills.
In response to NPR’s inquiry about whether they were considering following in the footsteps of the Republican attorneys general, the other pharmacy chains did not respond.
The case is targeting a federal agency so abortion rights advocates have said that a decision in favor of the plaintiffs will affect every corner of the country.
“If FDA approval of mifepristone is revoked, 64.5 million women of reproductive age in the US would lose access to medication abortion care, an exponential increase in harm overnight,” NARAL said in a statement in February, pointing to internal research.
The company said in a statement to NPR that it has responded to all of the attorneys general to assure them it won’t distribute mifepristone in their states.

