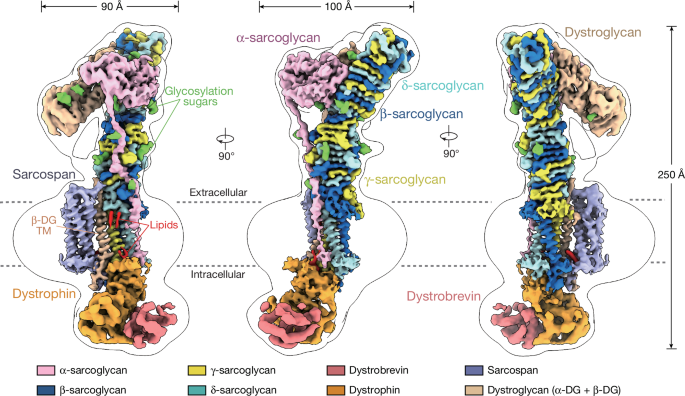
Muscular dystrophy-causing genes rationalized by the DGC structure
Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214-1221 (2020)
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Kimanius, D. et al. The size barrier of structure determination is reduced by data-driven regularization. Nat Methods 21, 1216-1221, doi:10.1038/s41592-024-02304-8 (2024).
Dystroglycan-agrin receptor association revealed by phosphorus in the cysteine-rich region of dystrophin: a possible novel target for skeletal muscle wasting
Swiderski, K. et al. Phosphorylation within the cysteine-rich region of dystrophin enhances its association with β-dystroglycan and identifies a potential novel therapeutic target for skeletal muscle wasting. That was a really, really, really hard thing to say. Mol. The person is related to Genet. 23, 6697–6711 (2014).
Ishikawa-Sakurai, M., Yoshida, M., Imamura, M., Davies, K. E. & Ozawa, E. ZZ domain is essentially required for the physiological binding of dystrophin and utrophin to β-dystroglycan. That’s terrible. Mol. It is Genet. 13, 693–702 (2004).
Deyst, K. A., Bowe, M. A., Leszyk, J. D. & Fallon, J. R. The α-dystroglycan-β-dystroglycan complex. The relationship to an agrin receptor is composed of two parts. J. Biol. Chem. 270 was published in 1995.
Esapa, C. T., G. R., Schroder, J E., Kroger, S. and Blake, D. J. The effects of post-translational processing on dystroglycan synthesis and trafficking. FEBS Lett. 555, 209–216 (2003).
The role of 7 integrin in muscular dystrophy: a review and meta-analysis on the epidemics of limb girdlers
Mah, J. K. et al. A review and meta-analysis on the epidemiology of muscular dystrophy. Neuromuscular Disord 24, 482–491 (2014).
Muthu, M., Richardson, K. A. & Sutherland-Smith, A. J. The crystal structures of dystrophin and utrophin spectrin repeats: implications for domain boundaries. PLoS ONE 7, e40066 (2012).
Ramaswamy, K. S. et al. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. The J. Physiol. is a journal.
Marshall, J. L. Dystrophin and utrophin expression require sarcospan: loss of α7 integrin exacerbates a newly discovered muscle phenotype in sarcospan-null mice. I think that’s terrible. The name of the journal is mol. The Genet. 21, 4368–4313 were published in the year 2012
Bonnemann, C. G. et al. β – The loss of the sarcoglycan complex is the cause of muscular dystrophy. Nat. Genet. 11, 266–273 (1995).
Geis, T. et al. There is a novel disorder called multicystic leucodystrophy associated with a Homozygous dystroglycanmutation. The neurosciences 14, 205–213 were published.
M. et al. The correlation between genetic analyses of limb-girdler muscular dystrophy in Saudi Arabia and Sudan and the impact on cellular respiration is related to PYROXD1 deficiency. Physiol. Genomics 50, 929–939 (2018).
Piccolo, F. et al. A founder mutation in the γ-sarcoglycan gene of Gypsies possibly predating their migration out of India. Hum. It’s called mol. There is a Genet. 5, 2019–2022 (1996).
Dai, Y. et al. A patient with a rare, mild and late age of onset muscular dystrophy-dystroglycanopathy was identified through whole exome Sequencing. J. Cell. The name is derived from the Latin word “mol”, meaning “matter.” Med. 23, 811–818 (2019).
Feng, J., Yan, J., Buzin, C. H., Towbin, J. A. & Sommer, S. S. Mutations in the dystrophin gene are associated with sporadic dilated cardiomyopathy. There is a word named “mol.” The person is Genet. Metab. 77, 119–126 (2002).
The sarcoglycan structure rationalizes muscular dystrophy-causing mutations and autoproteolysis of membrane-bound MUC1 mucin
Fraiberg, M., Borovok, I., Bayer, E. A., Weiner, R. M. & Lamed, R. Cadherin domains in the polysaccharide-degrading marine bacterium Saccharophagus degradans 2-40 are carbohydrate-binding modules. J. Bacteriol. The book is titled, “199, 283–155.”
Vickers, C. et al. Structural and mechanistic similarities exist between -L-fucosidases from GH 29 and the hydrolases of the glycoside hydrolase family 107. J. Biol. The Chem. 293 was published in 2018).
Macao, B., Johansson, D. G., Hansson, G. C. & Hard, T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat. Struct. 13, 71– 76.
Noguchi, S., Wakabayashi, E., Imamura, M., Yoshida, M. & Ozawa, E. Formation of sarcoglycan complex with differentiation in cultured myocytes. Eur. J. Biochem. 267, 640–648 (2000).
Source: Native DGC structure rationalizes muscular dystrophy-causing mutations
Crystal structure and role of -dystrobrevin in bacterial endosialidase of bacteriophage K1F
Stummeyer, K., Dickmanns, A., Muhlenhoff, M., Gerardy-Schahn, R. & Ficner, R. Crystal structure of the polysialic acid-degrading endosialidase of bacteriophage K1F. Nat. Struct. Mol. Biol. There were about 90 pages in the year of 2005.
Grady, R. M. et al. Tyrosine-phosphorylated and nonphosphorylated isoforms of α-dystrobrevin: roles in skeletal muscle and its neuromuscular and myotendinous junctions. J. Cell Biol. 160, 741–752 (2003).
Grady, R. M. et al. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin–glycoprotein complex. There was a Neuron 25 in 2000.
Ponting, D. J. J., Davies and K. E. were involved in the study of zinc fingers in dystrophin. Trends in medicine. The journalSci 21, 11– 13 was published in 1996.
Peter, A. K., Marshall, J. L. and R.H. were present. The J. Cell Biol. 183 was written in 2008.
Source: Native DGC structure rationalizes muscular dystrophy-causing mutations
DGC-based partner-protein recognition in Dag1-null mice: the role of dystrobrevin and sarcoplasmic reticulum
The structure, dynamics and principles of partner-protein recognition were explained by Susa, K.J. and others in the paper. A Cell Biol. https://doi.org/10.1016/j.tcb.2023.09.003 (2023).
R. A., and others, reported on a topic. Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum. There is a scientific term for this. It’s Genet. 6, 822–822 (1997).
Microspan is a novel component of the sarcoplasmic reticulum that causes severe muscle pathology. J. Muscle Res. Cell Motil. 27, 545–558 (2006).
M. Yoshida, et al. Biochemical evidence for association of dystrobrevin with the sarcoglycan–sarcospan complex as a basis for understanding sarcoglycanopathy. Hum. Mol. There is a genetic component to it. 9, 1033–1040, went down in 2000.
Source: Native DGC structure rationalizes muscular dystrophy-causing mutations
The crystal structure of Bordetella pertussis virulence factor P.69 pertactina: A new domain in cryo-electron microscopy
The structure of the pectate lyase C is a new domain. Science 260, 1503–1507 (1993).
Emsley, P., Charles, I. G., Fairweather, N. F. & Isaacs, N. W. Structure of Bordetella pertussis virulence factor P.69 pertactin. Nature 381, 90–92 (1996).
Petersen, T. N., Kauppinen, S. & Larsen, S. The crystal structure of rhamnogalacturonase A from Aspergillus aculeatus: a right-handed parallel β helix. Structure 5, 533–544 (1997).
Parvatiyar and M. S. collaborated on a book. The isoproterenol response is regulated by sarcospan. J. Am. Heart Assoc. 4, e002487.
Mitchell, R. D., Palade, P. & Fleischer, S. Purification of morphologically intact triad structures from skeletal muscle. J. Cell Biol. 96, 1008–1016 (1983).
Kimanius, D., Dong, L., Sharov, G., Nakane, T. & Scheres, S. H. W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
A method for determining particle orientations, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. 333 was reported in 721–745.
Source: Native DGC structure rationalizes muscular dystrophy-causing mutations
Nature. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3: An Accessible eBook for All-atom Validation
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature can be accessed through its website at http://www.nature.org//10.1389/s41586-026-07487-w
V. B., Chen, and others. All-atom structure validation is needed for macromolecular crystallography. The name of the book is Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).