The science of the case threatens access to the abortion drug
Facebook Post by Honeybee Health: “It Is Your Pharmacy Certified to Dispensate Misoprostol?” Comments on the FDA’s Adjustment to the REMS Program
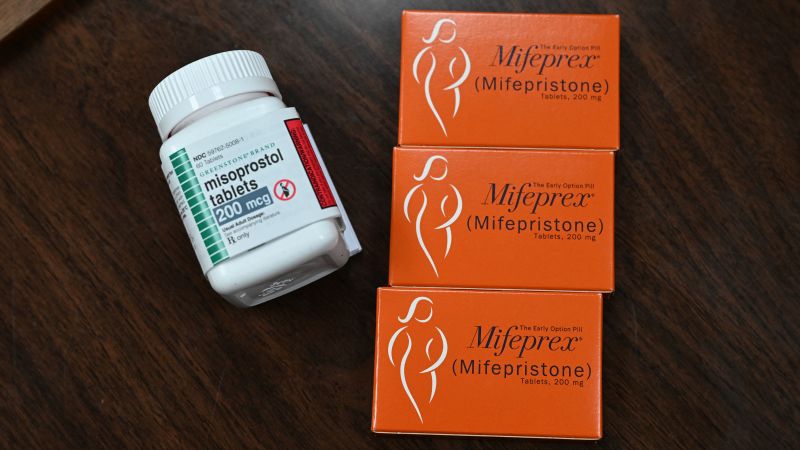
Honeybee Health, an online pharmacy company that had been supplying and shipping abortion medications, posted on its verified Facebook page Tuesday that it “officially became the first pharmacy certified to dispense medication abortion.”
For the first time, this allows outpatient pharmacies to dispense mifepristone, said Lewis Grossman, a professor of law at the American University Washington College of Law.
The answer is likely not, as I think it won’t make any difference in states where abortion is banned. “I don’t see any real effect there.”
Although the combination of mifepristone and misoprostol is the most commonly prescribed regimen for medication abortion in the United States, both the ACOG and the WHO say that using misoprostol by itself is a safe and effective alternative.
In a statement Wednesday, Walgreens said it is “working through the registration, necessary training of our pharmacists, as well as evaluating our pharmacy network in terms of where we normally dispense products that have extra FDA requirements and will dispense these consistent with federal and state laws.”
The first digital pharmacy to supply and ship abortion was Honeybee Health. We are proud to partner with the majority of telemedicine abortion providers in the US and to work closely with our manufacturer to help set the high standards required for certification in response to the FDA’s adjustment to the REMS program,” the Facebook post says. REMS refers to the Risk Evaluation and Mitigation Strategy program for mifepristone.
The FDA’s certification of mifepristone does not preempt state laws regulating medication abortion: An epidemiologist’s perspective
For many pharmacies, it may take them some time to review and make a decision about the certification process.
The FDA’s pharmacy certification for mifepristone requires pharmacies to track shipments and to keep records of prescribers, recipients and lot numbers of each drug dispensed. If you’re in a state with access, you can send a drug to people in states that don’t allow abortion.
It’s an option for medication abortion according to an epidemiologist who works at Ibis Reproductive Health, a global research organization that supports abortion rights. “It’s just that clinicians in the United States have less experience providing it, because for so long the default has been the combined regimen,” she says.
Miller praised the FDA’s changes, saying they are an important step forward in increasing access to abortion medication, but she said there is an unfortunate reality.
“We don’t believe that anyone should be forced to travel in that way, and certainly, as this moves forward, there are a lot of very smart lawyers who are looking at the question of how they’ll be incorporated into drugstores and pharmacy chains, and where that can happen – and how these different federal and state provisions interplay,” she said.
He wanted to know if a state that was prosecuting someone for diversion would have access to those records. Because if they do, then that is a disincentive to providing it to people in states that are banning it.”
In the current political climate, “it’s far from clear” whether the US Supreme Court would say the FDA’s recent actions “preempt” state laws restricting access to medication abortion, Litman said, or that state laws are preempted to the extent they regulate medication abortion.
“This really threatens the FDA’s authority over the approval process for medications across the board,” says Allen. The FDA is not the one who make these decisions, rather they are the ones with the scientific and medical expertise.
In response to the lawsuit, the FDA’s attorneys have written that mifepristone’s approval did not involve an accelerated review (the approval process took four years). They said that there isn’t a concrete example of a patient who might have suffered a serious adverse event associated with the drug. “That omission is particularly telling, given the more than two decades that mifepristone has been in use,” the court filing says.
Another reason why misoprostol isn’t often administered on its own is that some clinical trials have shown that it has a lower success rate, Moseson says. She said that it was probably due to differences in study design and local conditions. If a few days of taking misoprostol don’t make the abortion complete, participants are offered a surgical abortion. It would be marked as an unsuccessful use of the drug. The success rates are higher in settings where surgical intervention is not readily available.
If such a decision were to be appealed, it would land in the US Court of Appeals for the Fifth Circuit, “one of the most conservative appellate courts in the country”, according to Allen. Assuming that the court upheld this theoretical decision by Kacsmaryk to ban mifepristone, the next option would be for the FDA to take the case to the Supreme Court.

