A baby with a genetic disease has the world’s first personalized CRISPR therapy
Putting CAST into bacterial bacteriophage, and using it to improve the survival of a cancer-associated bacterial cell with gene-corrected therapies
The new tool promises to insert entire genes, precisely and efficiently, into human DNA, which is what original CRISPR systems have struggled to achieve.
Today, in Science1, a method was described that could allow for gene- correction therapies to be given once and work regardless of the specific flaw causing the disease. It could also accelerate the development of engineered cell therapies for cancer and simplify the creation of genetic models for research.
The co-author of the study, a chemical Biologist at the Broad Institute, said that it could be a big part of the future.
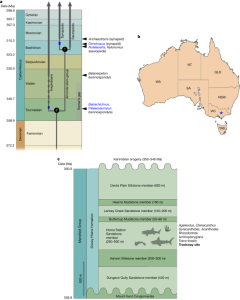
Transposases are enzymes that power the movement of ‘jumping genes’ — ‘selfish’ bits of DNA that hop around the genome to propagate themselves. Researchers have already repurposed CAST systems to shuffle large chunks of genetic material in bacterial cells. But in human and other mammalian cells, all previously reported versions of CAST have struggled with low efficiency.
directed evolution is a technique that uses the power of Darwinian selection in the laboratory to overcome barriers. They put the key genes encoding the components of a CAST system into a bacteriophage, a virus that infects bacteria. Their set-up ensured that such viruses with the most effective versions of CAST — those that integrated DNA into the genome quickly and accurately — would grow best.
The scuplture was a more than 400-fold improvement over the non-evolved original.
After undergoing three different treatments to correct a genetic condition that impaired his body’s ability to process protein, little KJ Muldoon is doing great, his parents told reporters. But it is too soon to use the word “cure”, says Rebecca Ahrens-Nicklas, a paediatrician at Children’s Hospital of Philadelphia in Pennsylvania, and one of Muldoon’s physicians. She says that it’s not yet really early days. We have more to learn from him.
In just six months, a group of clinicians and researchers from industry and academia came together to develop Muldoon’s therapy. The drug described in the New England Journal of Medicine is very specific to Muldoon’s genetics and will not be used for another person.
Muldoon did not produce the normal form of a key component of the body’s immune system because he had two different changes in his parents. He was unable to process the nitrogen compounds produced by the body. His blood contained high levels of ammonia, a compound that is harmful to the brain.
The best treatment for CPS-1 deficiency is a liver transplant, but it would be months before Muldoon became eligible. Only about half of babies with a serious deficiency in CPS-1 survive long enough to receive a transplant.