There are sex toys called sphinxes that are genital vi brotactile sensors
Labelling of TrkB+ and Ret+ populations using tamoxifen in a reverse light-dark cycle room
At least 2 weeks before testing, mice were transferred to a reverse 12 h–12 h light–dark cycle room. The mice were 2 months old when they were used. For two generations they were from a mixed background, but reverted back to C57Bl/6 mice.
Tamoxifen was dissolved in 100% ethanol, diluted 1:2 in sunflower seed oil and vacuum-centrifuged for 30 min. The TrkB+ population was labelled densely for immunohistochemistry and using an injection of a small amount of tamoxifen at P5 on top of it. In a few cases, the pregnant mother was given tamoxifen at E 16.5 or E15.5 in order to label them with the same populations. The Ret+ population was densely labelled with 3 mg of tamoxifen delivered to the pregnant mother through oral gavage at E11.5 or E12.5 and sparsely with 0.5 mg at E12.5.
Source: Krause corpuscles are genital vibrotactile sensors for sexual behaviours
Transverse cryosection of the spinal cord and genital tissue using a cryostat (Leica) for the isolated urethra, cervical vertebra and spinal cord
After removal of the musculature and the overlying submandibular nerve, the DRGs and spine were isolated by means of a surgical instrument. Penile tissue was isolated from the preputial glands and external and internal prepuces, and the proximal portion of the penis was discarded. Working proximal to distal, the clitoris was isolated from perineal tissue by removal of remaining pelvic musculature and preputial glands, then by fine dissection of the clitoris and urethra from above the vaginal canal and inside of the external prepuce. The digit-tips were cut to make them stain the paws.
Tissue was cryoprotected in 30% sucrose in 1× PBS at 4 °C overnight, embedded in Neg-50 and frozen at −80 °C. Spinal cord and DRG samples were sectioned transversely using a cryostat (Leica) at 30 µm and placed onto slides. To make the sectioning easier, genital tissue and digit-tips were sectioned at 30 m thickness and put into a well plate with 1 PBS. Thin cryosections were allowed to dry overnight. The sections were rehydrated with 1× PBS, blocked with 5% normal donkey serum in 0.1% PBST (0.1% Triton X-100 in 1× PBS) for 2 h at room temperature, and incubated with primary antibodies in blocking solution for 2 days at 4 °C. The slides were rinsed in PBST three times for 10 min and incubated with secondary antibodies in blocking solution overnight at 4 °C. The slides were mounted on a mount and then washed in PBST.
The main antibodies used in the study were chicken, goat and goat anti-mCherry.
Analysis of penis and other neuronal data using multiple unpaired t-tests and Tukey’s multiple-comparisons test
The analysis was conducted using unpaired t-tests, one-way ANOVA with post hoc multiple unpaired t-tests, two-way ANOVA with Tukey’s multiple-comparisons test and Fisher’s exact tests. All P values for unpaired t-test used are two tailed. The exact P values and other statistics for unpaired t-tests, multiple unpaired t-tests or Tukey’s multiple-comparison test used in the study are as follows: in Fig. 1g, P = 0.9355, t = 0.085, d.f. = 5, F = 1.002; Fig. 1i, between clitoris and glans penis, P < 0.000001, t = 13.66, d.f. = 917, F = 2.514; between clitoris and digit (Meissner), P < 0.000001, t = 6.82, d.f. = 682, F = 8.169; Fig. 2f, for datapoints of penis, P = 0.001138, t = 3.482, d.f. = 44; for datapoints of digit, P = 0.000224, t = 4.068, d.f. = 39; for datapoints of clitoris, P = 0.000104, t = 4.409, d.f. = 33; Fig. 2g, for datapoints of penis, P = 0.002853, r = 3.156, d.f. = 45; for datapoints of digit, P = 0.8476, t = 0.1934, d.f. = 41; for datapoints of clitoris, P = 0.00666, t = 2.885, d.f. = 35; Fig. 4i, between clitoris and penis in the TrkB+ neurons, P = 0.000001; between clitoris and penis in the Ret+ neurons, P = 0.2023; between TrkB+ and Ret+ neurons of clitoris, P < 0.000001; between TrkB+ and Ret+ neurons of penis, P = 0.000632; Fig. 5g, for session 1, P = 0.6457, t = 0.08194, d.f. = 15; for session 2, P = 0.6358, t = 0.4834, d.f. = 15; for session 3, P = 0.6994, t = 0.8235, d.f. = 15; Fig. 5h, for session 1, P = 0.8526, t = 0.1891, d.f. = 15; for session 2, P = 0.3264, t = 1.015, d.f. = 15; for session 3, P = 0.5408, t = 0.6259, d.f. = 15; Fig. 5i, for session 1, P = 0.008921, t = 3.117, d.f. = 11.99; for session 2, P = 0.003970, t = 3.453, d.f. = 13.76; for session 3, P = 0.004659, t = 3.344, d.f. = 14.412; Fig. 5k, P = 0.0126, t = 2.858, d.f. = 14, F = 17.18; Fig. 5l, P = 0.0026, t = 3.558, d.f. = 16, F = 2.473; Fig. 5m, P = 0.0009, t = 4.064, d.f. = 16, F = 1.133; Extended Data Fig. 1e, P < 0.000001, t = 7.991, d.f. = 20, F = 2.293; Extended Data Fig. 8c, for cups, P is 0.8336, T is 0.2148, d.f. For different Interstimulus intervals from small to large of females, and the same Interstimulus interval from male to male, P equals 0.6498, 0.8012, 0.1837, 0.2384, and 0.5429. 9b, for females, P = 0.7969, t = 0.2619, d.f. = 15, F = 1.409; for males, P = 0.6961, t = 0.3970, d.f. = 18, F = 3.950; Extended Data Fig. 9d, for number of mounts, P = 0.4617, t = 0.7416, d.f. = 49, F = 1.445; for mount total duration, P = 0.4876, t = 0.6994, d.f. = 49, F = 1.865; for mounting start time, P = 0.0064, t = 2.850, d.f. = 49, F = 6.615; Extended Data Fig. 9e, for ejaculation freeze, P is 0.30, t is 1.072. 9f, for intromission number, P = 0.8697, t = 0.1649, d.f. = 45, F = 1.531; for intromission start time, P = 0.0287, t = 2.250, d.f. = 53, F = 1.717; for intromission total duration, P = 0.0252, t = 2.318, d.f. = 45, F = 3.553; for intromission duration per bout, P = 0.000003, t = 5.324, d.f. = 45, F = 2.618; for inter-intromission interval, P = 0.0222, t = 2.370, d.f. = 45, F = 9.020; Extended Data Fig. 9g, from trial 1 to 3, P = 0.2473, 0.4192, 0.1055, t = 1.216, 0.8324, 1.723, d.f. = 12, 14, 15; Extended Data Fig. 9h, from trial 1 to 3, P = 0.8998, 0.7724, 0.01131, t = 0.1287, 0.2949, 2.886, d.f. = 12, 14, 15; Extended Data Fig. 9i, from trial 1 to 3, P does not have a value; between trial 1 and 3 does. P from trial 1 to 2 is 0.2149, 0.9918, and 1.286, d.f. 10i, P, is 0.0033, T, is 3.455, and d.f. is 16, F, is 4.197
Complex Krause corpuscles were defined as Krause corpuscles containing tightly coiled axons. Complex Krause corpuscles often exhibited globular shapes, while some also exhibited elongated shapes with convoluted axonal profiles. The Krause corpuscles were defined as having 1 or 2 linear axons. The shape of all simple corpuscles is very similar to the shape of the Pacinian ones but with a smaller size.
Source: Krause corpuscles are genital vibrotactile sensors for sexual behaviours
Imaging of nerve terminals using a novel mouse model using an epoxy resin, ladd Research, and a molecule-infiltrated microscopy
The samples were smicated with 1% of osmium tetroxide/1.6% of kantha ferrocyanide. The section were washed with double distilled H2O and stained with 1% uranyl acetate at 4 C. The sections were dehydrated after washing with double-distilled H2O. The sections were then infiltrated with 1:1 mix of epoxy resin (LX-112, Ladd Research) and propylene oxide at 4 °C overnight. The sections were cured at 60 C for 48–72 hours after they were embedded. Ultrathin (approximately 60 nm) sections were generated and imaged on the JEOL 1200EX transmission electron microscope at 80 kV accelerating voltage. The images have been cropped.
There were single nerve terminals in genital and glabrous tissue which could be visualized using the Brn 3acKOAP placental AP reporter mouse55. C-LTPRs were densely labelled with the drugs Th2A-creER;Brn3acKOAP. At three weeks old, it was administered. Although sympathetic fibres are TH+, they do not express BRN3A; thus, sympathetic fibres are not labelled in TH2A-creER;Brn3acKOAP animals. The MRGPRB6+ afferents were labelled using the injections of AAV-FLEX-PLAP64 into the mice. All of the mice were rejected after 7 weeks of age.
The post-fixed and dissected tissue was incubated in PBS at 68 °C for 2 h to inactivate endogenous AP, then rinsed three times for 5 min in B3 buffer (0.1 M Tris pH 9.5, 0.1 M NaCl, 50 mM MgCl2, 0.1% Tween-20) at room temperature. tissue samples were placed in a B3 buffer with 3.4 l of each for the PLAP reaction. Stained tissue was then pinned flat in a dish, fixed in 4% PFA in PBS for 1 h at room temperature and serially dehydrated in ethanol (50%, 75%, 100%, 1 h each, then 100% overnight) while covered. The image of the tissue was done using a stereoscope.
Source: Krause corpuscles are genital vibrotactile sensors for sexual behaviours
Neuron arborizations as sensor systems for genital vibrotactile sensing: Injection of CTB or AAV using glass pipette
Individual neuron arborizations were imaged for analysis. The terminal area was measured in ImageJ by drawing a tight polygon around the terminals of a given axon, and the number of corpuscles innervated by each fibre was counted manually. It is possible that the corpuscles of a given afferent occupy a larger volume in the z dimension than is measured in this manner. Simple and complex corpuscles had different types of terminals that were defined as thin and linear. Reconstruction and filling of representative fibres was performed using the ImageJ SNT plugin.
Young adult mice were injected with isoflurane for 5 minutes from a precision vapourizer in order to get them a good night’s sleep. Injections were done with a glass pipette. The glass pipette was connected to an aspirator tube assembly (Sigma-Aldrich, A5177-5EA), which was connected to a syringe to control the air pressure.
The hairs near the male or female genital area were removed for injection of mid-line hairy skin through a method called Nair treatment and then cleaning using 70% alcohol. A pair of blunt instruments was use to hold the hairy skin in place and insert the glass pipette. After a successful injection, the fast green dye was immediately visible within the hairy skin region during the injection.
A total volume of 2 μl was injected into either the male or female target region, for injection of either CTB or AAV. Animals of 4 weeks old were used for the injections. For AAV injections, 3–4 week old animals were used, AAV2-retro-hSyn-FlpO (4.95 × 1013, Boston Children’s Hospital Viral Core) was used, and the injected animals were perfused after 3 weeks.
Source: Krause corpuscles are genital vibrotactile sensors for sexual behaviours
Using JRCLUST to identify levels of the lumbosacral spine sections in mice and study the effect of nerve cells activated by vibrations
The ratio of the Ld to the central of the lumbosacral spinal cord sections was decided by the characteristic decrease in the size of the dorsal column, from the lower back to the more modern areas of the spine. Lc was defined as the distance between the middle of the inner surface of the brain and the central canal. The ratio of Ld/Lc was calculated and used for the reference to identify the levels of the lumbosacral sections.
The platform was moved to a different location. A 32-channel silicon probe (Cambridge NeuroTech, ASSY-37 H6b) was inserted into either the left or right L6 DRG. The signals were amplified and recorded using an Intan Technologies RHD2132 amplifier chip and RHD USB Interface Board. Open-sourced software was used to control data acquisition.
Specific nerve cells on the penis and clitoris detect vibrations and then become activated, causing sexual behaviours such as erections, a study in mice has revealed1. There could be new treatments for conditions such as Erectile Function Problems and People with Lower Body Paralysis because of the findings.
JRCLUST was used to sort action potentials into clusters and manually classify them into single or multi units.
Mechanical thresholds and sticky-tape assay for mouse hairy skin sensitivity determined by time-of-spike studies of optotagged TrkB+ neurons
To calculate the conduction velocity of optotagged neurons, the latency was determined by subtracting the time of each spike from the middle point of each light pulse. The latencies for the four afferents were 8.6 ms, 8.2 ms and 13.6 ms. The length of axonal projections from L6 DRG to the genitalia in adult mice was estimated to be 5 cm. Thus, the conduction velocities of the four optotagged TrkB+ neurons are 11.4, 10.4, 6.4 and 3.7 m s−1 respectively.
The time of the first spike in response to the vibration was determined to determine the mechanical thresholds. The corresponding recorded force at that timepoint was defined as the mechanical threshold for that trial. Thresholds of the trials at each Frequency were averaged.
The surgery procedure for male mice was the same as for the MEA recordings above. The platform was moved to an upright EpiPlan microscope with a 10 air objective, after it had been exposed. The light source was a 470 nm LED (M470L5, Thorlabs) with an LED driver (LEDD1B, Thorlabs), and a CMOS camera (CS505MU1, Thorlabs) was triggered at 10 frames per second with 50 ms exposure time using ThorCam software (v.3.7.0). All stimuli were synchronized with the camera using a data-acquisition board (National Instrument, NI USB-6343).
The penis was thermal stimulated using a water refilling device. The room temperature, ice water, hot water, and the water baths were all connected to this device. The penis was submerged in the water reservoir. Then, water of different temperatures was pumped into the reservoir at a controlled speed, and room-temperature water was used during the baseline measurements. There was a collection chamber beneath the reservoir that was where the water came from. The temperature was measured using a pair of instruments: a microprobe (IT1E, Physitemp) and a thermometer (BAT-12).
The sticky-tape assay as a measure of hairy skin sensitivity was performed using a previously described protocol67. There was a piece of laboratory tape secured to the animal. After entering the enclosure, the animal was video-monitored for a period of 5 minutes. The attempts to remove the tape were quantified using a software that counts shaking, biting, and scratching.
According to a previous procedure, the tactile prepulse inhibition experiments were conducted. The cylinder holding the mouse has a 3.8 cm diameter. The container was locked up in the soundproof chamber. The prepulse was delivered at various intervals to the back of the animal before an acoustic startle. The animal had a startle response that was recorded. PPI was calculated as %PPI = [1 − (startle response from pulse following prepulse/startle response from pulse alone)] × 100.
The strain gauge amplifier connected to a load cell was used to measure force. Vibratory stimuli were administered directly onto the load cell. The glans penis was moved across a piece of vaginal tissue that was put in a load cell for a simulation of genital contact. After the noise was reduced with the Hum Bug noise Eliminator, the data was downloaded using the Digidata 1550B system. The power spectrum analyses were performed with a sample taken from either the baseline or stimulation phases.
Source: Krause corpuscles are genital vibrotactile sensors for sexual behaviours
In silico optical stimulation of TrkBcreER mice: An animal study of the success of sciatic nerve dissection using Isoflurane through a nose cone
The rats were snoozing with isoflurane through a nose cone. The bladder was emptied using gentle abdominal pressure. Body temperature was maintained at 37 ± 0.5 °C using a heating pad, and ophthalmic ointment was placed on the eyes. The back was shaved and sterile after reflexes were absent. The T9 and T10 spine segments were exposed by the midline and paravertebral musculature cuts. The scalpel blade was moved to ensure thorough transection after it was inserted between the T9 and T10 spinal segments. Bleeding was controlled with sponges and cotton. The skin of the back was bandaged up in order to allow mice to recover. The success of the sciatic nerve dissection was confirmed by hindlimb paralysis. Food and DietGel were placed onto the floor of the cage, and carprofen (5 mg per kg) was injected subcutaneously every 24 h. Bladder expression was performed every 2–3 h on the day of surgery and every 6–8 h on the next day. The mice were put down soon after the injury to their spine.
For mechanically induced sexual reflex behaviours, 2–4-month-old mice were used. For the optogenetic stimulation of TrkBcreER mice, 4–6-month-old animals were used because externalization of the glans penis was not feasible in younger animals that received tamoxifen treatments at P5. Red light was used to illuminate the set-up during optogenetic induced behaviours, while room light was used for mechanical stimulation measurements. The videos were taken at 60 frames per second. The start and end of each sexual function were quantified using a software. The behavioral measurements were analysed and plotted in a computer program.
Female mice were given 17-estradiol bucoate two days before the test. It’s helpful to have access to the vaginal opening. The day prior to testing, the mice were transected at the T9/T10 level using methods described above. On the day of the test, the awake, paralysed animals were gently restrained in a supine position with all four limbs taped on a platform, to reduce motion artifacts during the pressure measurements. The latex balloon (Harvard Apparatus, 73-3813) was attached to a cannula and secured with a tape on the platform. The pressure transducer was connected to the assembly through the tubing. The output was amplified and digitalized with the help of AcqKnowledge software and the BIOPAC MP150 system.
The videos of mating behaviours were manually scored using BORIS software69 by an experimenter who was blinded to the genotype of the animals. For male mating behavioural analysis, the following behaviours were scored: sniffing, mounting, intromission and ejaculation. Mounting refers to the males’ rapid pursuit of females, followed by grasping their rear, and often followed by short probing without gaining access to the female genitalia. Intromission represents the long rhythmic thrusting, indicating the male’s successful penetration of the vagina. The mounting and intromission periods are not related to the scoring methods used. The bouts lasting under 2 s were excluded from the analysis because of the lack of rhythm and the possibility that penetration did not occur. Moreover, combative behaviours and darting behaviours of female mice were scored during the female mating behaviour assessment trials. There are instances in which a female fights with a male while there are also instances in which a female tries to escape from a male.
Data were analysed using MATLAB (v.2019a or v.2021a), Python (v.3.7.7) and GraphPad Prism 10. The number of samples and statistical tests used for individual experiments can be included in the figure legends. In Fig. 2c, n = 10 sections from 3 control females, 10 sections from 3 TrkBcKO females, 7 sections from 2 control males and 20 sections from four TrkBcKO males. In Fig. 2f, n = 32 TrkB+ axons from 5 males, 16 Ret+ axons from 3 males, 26 TrkB+ axons from 4 females, 11 Ret+ axons from 3 females, 26 TrkB+ axons from 4 animals’ digits, and 17 Ret+ axons from 4 animals’ digits.
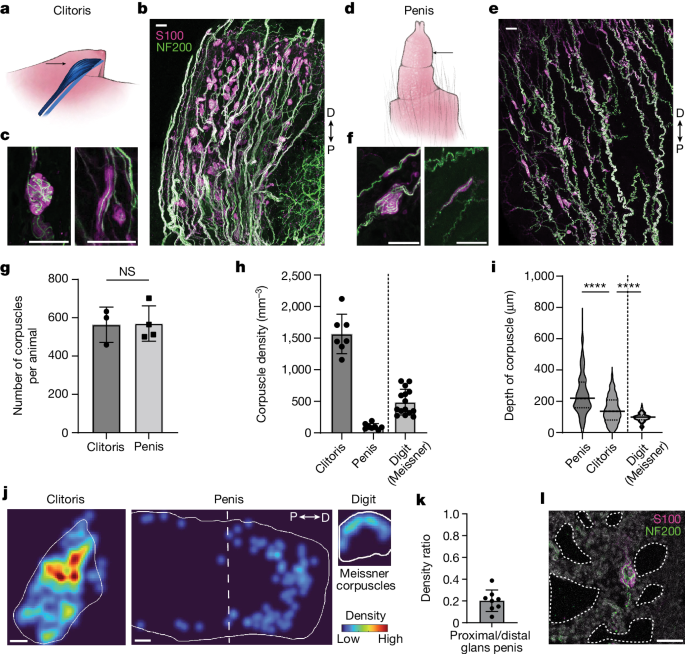
Krause corpuscles — nerve endings in tightly wrapped balls located just under the skin — were first discovered in human genitals more than 150 years ago. The structures are similar to touch-activated corpuscles found on peoples’ fingers and hands, which respond to the shaking of a textured surface.
Ginty and other sensory biologists have long wanted to study these mysterious neuron balls. It was almost impossible to use advanced molecular techniques to track and activated specific neurons in the past.
The researchers found that the mice didn’t develop the cortex until 6 weeks after they were old enough to have sex. The team is studying how hormones in the female mouse’s oestrus cycle affect the function of the corpuscles, as well as how these late developing neural systems wire themselves into the body’s existing nervous system.
A paper his group published in the year of 2016 showed that a touch-sensitive pathway in the genitals is necessary for successful sex. Sex is one of the major drivers of evolution, and it is a fundamental area of biology. He hopes that further research into these nerve cells will eventually lead to treatments for conditions such as erectile dysfunction and vaginal pain.