Parkinson’s symptoms improved in the stem-cells trials
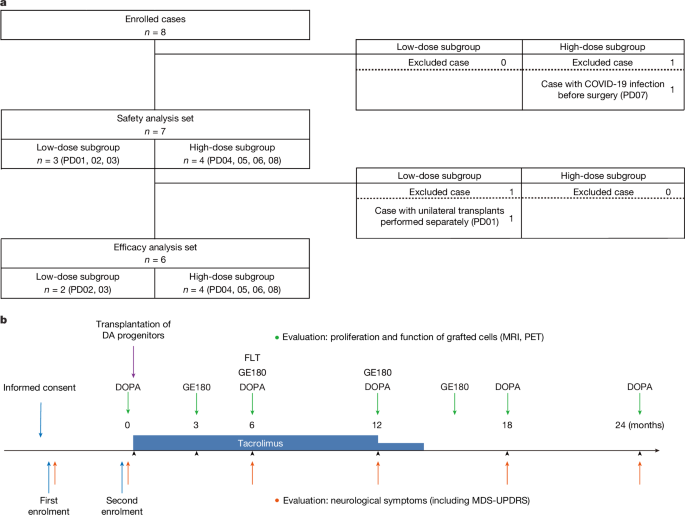
Safety profile of allogeneic transplantation of iPS-cell-derived DA progenitors in patients with PD and implications for cell transplant therapy
In conclusion, while the safety and efficacy of iPS-cell-derived cell products continue to be investigated, this trial demonstrated the safety profile of iPS-cell-derived DA progenitors. The average motor severity was lowered and the mean 18F-DOPA uptake was increased after bilateral putaminal transplantation. Despite the abovementioned limitations, these findings suggest that allogeneic transplantation of iPS-cell-derived DA progenitors is a safe and effective regenerative therapy for patients with PD. Future strategies could combine cell transplant with other treatments to improve efficacy. A single case study8 showed that autologous transplantation using iPS cells can be a promising option.
Results from March suggest that for three people who received the treatment, the cells survived and are safe one year after surgery. But the signs of benefit are mixed. One of the three people said that she could now see her husband’s face, but only through a small part of her eye, which had been damaged in a car accident.
The trials were mainly designed to test safety and were small, involving 19 individuals in total, which is not enough to indicate whether the intervention is effective, says Parmar.
“Some people got slightly better and others didn’t get worse,” says Jeanne Loring, a stem-cell researcher at Scripps Research in La Jolla, California, which could be due to the relatively small number of cells transplanted in these first early-stage trials.
Dopamine Production in the Neurons of Parkinson’s Disease Overcame the Years of a Surgery using Stem Cell Transplants
Parkinson’s is caused by the loss of dopamine-rich braincells, which cause tremors, rigidity and slow movement. It is not safe to say that there will be 25 million people who suffer from the condition by mid-century.
Stem cells were injected to 18 places across the putamen in both hemispheres, to fill up that region of the brain.
Five individuals received a dose of 0.9 million cells and 7 received 2.7 million cells, in the hope that 100,000 and 300,000 cells, respectively, would survive the surgery. A healthy brain typically has 300,000 dopamine-producing neurons. The recipients were given immune-suppressing drugs for one year after the surgery to prevent their bodies from rejecting the transplant.
Brain scans showed an overall increase in dopamine production, suggesting that some neurons survived the entire 18-month observation period, even after the participants stopped receiving immune-suppressing drugs.
On average, individuals who received the low dose showed a 9-point improvement in their symptoms on a standardized assessment for Parkinson’s disease, and those who received the high dose gained 23 points. The assessment measures individuals’ daily life activities, pain levels, sleep and eating. Agnete Kirkeby, a stem-cell scientist at the University of Copenhagen who is involved in a European trial, points out that this metric is somewhat subjective and can be influenced by the placebo effect, but says the results warrant larger trials.
Development of pluripotent stem cells based on adult cells: A Japanese experiment to improve the survival rate of iPS cells for a low survival rate on stem cell transplants
Yamanaka’s lab at Kyoto University discovered in 2006 that adult cells can be reprogrammed into stem cells, which can be used to make virtually any kind of tissue. These induced pluripotent stem cells — or iPS cells — won Yamanaka the Nobel Prize in Physiology or Medicine in 2012, and propelled him to superstar status. They have become a symbol of the country’s global scientific aspirations.
Three individuals received up to 5 million cells and 4 received up to 11 million cells, of which 150,000 and 300,000 cells, respectively, were expected to survive. “This low survival rate is a big problem that needs to be solved,” says Jun Takahashi, a neurosurgeon at Kyoto University in Japan, who led the trial. Participants were given immune-suppressing drugs for 15 months.
Only one of the three patients who exhibited a beneficial effect did not show improvement in the off-time period. These evaluations reflect the patient’s perceptions, and are subjective. It is possible that patients had very high expectations for this new treatment, and the results did not meet such high expectations despite objective improvements. In the remaining two patients (PD05 and PD06), motor deficits stabilized at a similar level of decline to those receiving conventional medication36,37. These two patients exhibited a higher degree of deterioration in motor symptoms compared with the other patients during the on-time, suggesting that faster neurodegeneration, especially in the non-dopaminergic systems, diminished the beneficial effects produced by the graft during the trial period. Notably, PD05 was 69 years old at the baseline, and as previous studies on fetal transplants suggest4,5, younger patients with less severe symptoms appear to be more suitable candidates for this treatment. Considering these results, refining patient eligibility criteria may enhance the efficacy of this treatment.
In some cases, especially PD03 and PD06, discrepancies were noted between the MDS-UPDRS part III scores and the Hoehn–Yahr stage. The Hoehn–Yahal stage is more concerned with instability and mobility issues, whereas the MDS-UPDRS part III includes more comprehensive evaluations of major motor symptoms. Consequently, improved postural stability and mobility may account for the greater improvement in the Hoehn–Yahr stage compared with changes observed in MDS-UPDRS part III scores of this study.
Masayo Takahashi: Taking the First Step to Provide Treatment of a Patient with Transplantable Tissues from iPS Cells
Scientists launched clinical trials and start-up firms. Large companies invested more in manufacturing hubs. Medical facilities are ready to receive patients from Japan and abroad. Masayo Takahashi, president of Vision Care, says thatgenerative medicine in Japan is moving very quickly. In 2014, she became the first to treat someone with cells derived from iPS cells.
There is a need for both policymakers and the public to be aware that careful evaluation of new science-based medical products is best for patients, researchers and organizations taking such interventions to the clinic. Regenerative medicine is an exciting and promising science, and it has taken researchers decades to bring it to the point of clinical application. Regulators around the world must not put that promise at risk by rushing the final stage of the process.
But those approvals are not yet in hand, treatment costs are high, large trials showing clear clinical benefit have yet to materialize, and concerns about safety could still sap the public’s willingness to try this treatment. We have to realize what the cells can do and what they can’t.
Yamanaka’s iPS cells promised to bypass a bioethical stand-off that had threatened the potential of embryonic stem cells for a decade. iPS cells do not need to be destroyed in order to production, so they were considered less fraught. They promised to offer transplantable tissues without the need for immune-Suppressing drugs because they could be made from the cells of the person in need.
In 2014, Takahashi put this idea to the test. She took skin cells from a 70-year-old woman with a progressive eye condition known as macular degeneration and guided them into a younger, more pliable state using a recipe similar to the one Yamanaka had devised and refined. The cells from the iPS cells were grown in a petri dish and used to grow new cells in the woman’s eye, preventing vision loss for ten years.
It was a procedure with practical limitations, however. The large cell-sheets that were crafted for implantation required intrusive surgery, and self-derived cell therapies are time-Consuming and expensive to make. Takahashi says she chose this approach to ensure the highest chance of clinical benefit — to demonstrate to the world what was possible. It was meant to be the best treatment.
The difficulties might come down to the retina’s natural resistance to regeneration. But other parts of the eye might benefit more from cell therapies: the cornea, the clear covering that lets light in, is maintained by a pool of stem cells and constantly being rebuilt.
The need for expedient approvals in Parkinson’s disease research in Japan: The case of the Center for iPS Cell Research and Application
Nishida has since set up a start-up company, Raymei, which plans to launch a larger trial and aims to gain formal approval in three years. The next trial is very important according to him.
The Center for iPS Cell Research and Application (CiRA) is an institute established by Yamanaka as a hub for iPS-cell research and is headed by a neurosurgeon.
Even without approvals in hand, the industry is building capacity in the expectation that demand for these treatments will be high. The world’s first manufacturing facility for donor derived iPS-cell products was completed by Sumitomo Pharma in the year 2018, he said. A building in Osaka looks like a silver box. In 2020, it delivered its first cells for transplant — for the fourth participant in Takahashi’s Parkinson’s trial. The company is also supporting two early-stage Parkinson’s trials in the United States.
Others are not worried about Japan’s fast-track process for rare conditions. In order to move the field forward quickly you need to have a degree of risk. Japan is putting regulations in place.
The national health system mostly covers the treatments that companies are able to offer. They have to continue to collect data in order to get full clinical approval.
The fast-track system’s safeguards are said to be working. But at least two of the candidate treatments clearly needed more time in clinical trials. Patients received false hope and taxpayers were saddled with an unnecessary bill because treatments grantedconditional approval are paid for through Japan’s public health insurance system.
Machine learning-based manufacturing of a white, muscular-looking, two-armed robot to evaluate macular-degeneration treatments for transplantation into human cells
Masayo Takahashi has chosen a more portable manufacturing model for her macular-degeneration treatments: a white, muscular-looking, two-armed robot. As the cells are being prepared for transplant through a microscope, it checks their progress with machine learning. In 4 months, it can produce enough cells for more than 800 individual treatments.
It is important that the therapies do not move into the clinic too quickly as they have the potential to change lives. Researchers must be allowed to take as much time as is necessary to complete safety and efficacy tests.
The results of both of the studies that are reporting the results of early stage clinical trials show that the interventions were safe, and that the recipients experienced some measurable improvements in their symptoms.