Immune evasion through transfer of mitochondria in the body
Cancer TILs are more likely to divide, and so are they? A study of Mitochondrial Transfer in Bone Tumours
Mitochondria are energy-providing organelles found in nearly all cells of humans and in a variety of other species. It has become apparent over the last two decades that many cell types can transfer their mitochondria to other cell types. The process of horizontal or intercellular mitochondria transfer can be implicated in the maintenance of tissue health but can also contribute to cancer. Writing in Nature, Ikeda et al.2 report that cancer cells obtain functional mitochondria from immune cells called T cells that infiltrate the tumour. The T cells are less effective in protecting themselves against cancer because the cancer cells return their main energy source to the other side. This bidirectional exchange of mitochondria enables cancer cells to support their metabolic needs while simultaneously creating favourable conditions for tumour growth.
J.R.B. is a member of the Scientific Advisory Board, receives research support, and has consulted for Columbus Instruments within the past year.
Mitochondrial transfer is a study at the Washington University School of Medicine in St. Louis, Missouri.
“My first thought was that this sounds crazy, like science fiction. “But they seem to have the data for it,” says Maecker, who was not involved in the research. This is potentially a completely new biology that we weren’t looking at.
Tainted TILs were less able to divide and more likely to commit cell ‘suicide’, the team showed in cellular models. T cell exhaustion was seen in the mice with cancer who had TILs that had infused alien mitochondria.
A comparison of in-live and in-vivo mouse experiments on tHe using the Kaplan-Meier method and the log-rank test
The consistent results of the in tHe experiments were produced when they were biologically repeated three to four times. Consistent results were produced from all in-live mouse experiments, which included four to six mice per group.
The groups were compared with Fisher’s exact tests. The relationships between variables in different groups were compared using one-way ANOVA. Tumour volume curves were compared using a two-way ANOVA. Bonferroni correction was used for multiple testing. Progression-free survival and overall survival were defined as the time intervals from the initiation of anti-PD-1 monoclonal antibody therapy until the first observation of disease progression or death from any cause, and until death from any cause, respectively. The Kaplan–Meier method was used to analyse survival curves, and the log-rank test was used to compare groups. The tests weretailed by a significance level of P 0.05. GraphPad Prism 9 was used for the statistical analyses. Some bars show the means and standard errors of the means.
Source: Immune evasion through mitochondrial transfer in the tumour microenvironment
CD8+ T cell transfer experiments for a humane end point at Okayama University and its approval by the Japanese Ministry of Environment and Science
Cell lysates from MEL02, MEL04, MCF7 and MDA-MB-231 cells, EV lysates from MEL02 and MEL04 cells, and medium used for the culture of MEL02 and MEL04 cells were separated by SDS–PAGE and blotted onto polyvinylidene fluoride membranes (Merck Millipore). The membranes were blocked and then incubated with primary antibodies. After washing, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Finally, the bands were detected using Clarity Western ECL substrate (Bio-Rad) or ImmunoStar LD (Wako), and confirmed using a LAS4000 system (Cytiva). The manufacturer’s instruments were used to adjust the concentrations in each sample according to the Pierce BCA kits that came with them.
We also created adoptive T cell transfer models, using B6 mice. The CD8+ T cells from the immune cells of C57BL/6J or OT-1 mice were transferred into the SCID mice. Tumours were collected for evaluation after 28 days.
All mice were maintained under specific pathogen-free conditions at the animal facility of the Institute of Biophysics (Chiba Cancer Center Research Institute and Okayama University). Mouse experiments were approved by the committee for animal experimentation at the Okayama University and they met the US public health service policy on humane care of laboratory animals. The mice were killed as a humane end point if the maximum diameter of the tumours exceeded 20mm. The experimental schematics are summarized in Supplementary Fig. 3.
Female C57BL/6J mice (6–8 weeks old) were purchased from SLC Japan. C57BL/6J- Prkdc
Source: Immune evasion through mitochondrial transfer in the tumour microenvironment
In Vitro Cellular Proliferation based on Annexin V and eBioscience Fixable Viability Dye. eFluor for Live/Dead Cell Staining
Twenty-four hours after cells (103) were passaged on 96-well plates, in vitro cellular proliferation was evaluated using an IncuCyte ZOOM System (Essen BioScience) every 6 h for 48 h.
Apoptosis was evaluated by combining Annexin V (Thermo Fisher Scientific) and eBioscience Fixable Viability There is a Dye. eFluor (Thermo Fisher Scientific) for live/dead cell staining. According to the manufacturer’s instructions, each cell was incubated with Annexin V and eFluor for 15 min at room temperature and then analysed by flow cytometry. Cellular proliferation was assessed on the basis of the dilution of cells labelled with carboxyfluorescein succinimidyl ester (CFSE) using a CFSE Cell Proliferation kit (Thermo Fisher Scientific) and flow cytometry. Cells were washed 3 times with RPMI medium, and put into 10 M CFSE for a period of 20 min at 37 C in 5% CO2, then put into 3 days of live and dead cell staining.
The kit was used to assess cellular senescence according to the instructions from the manufacturer. In brief, cells were incubated with bafilomycin A1 for 1 h at 37 °C in 5% CO2, then incubated with SPiDER-β-Gal for 30 min and analysed by flow cytometry.
The manufacturer said that the Metabolic analyses were done using a flux analyser. 1.0 105 cells were sown in the supplemented Seahorse XFI medium with 1 mM pyruvate, 2 mM glutamine and 10 mM glucose followed by the poly-d-lysine coated XFp miniplates. The plate was then equilibrated at 37 °C in an incubator without CO2 for 40 min. Oxygen consumption rate was evaluated with injections of monoaminetetraacetic acid (1 M), FCCP (0 M) and rotenone–antimycin A (0 M). The extracellular acidification rate was evaluated by sequential injections of glucose (10 mM), oligomycin (1 μM) and 2-deoxy-glucose (50 mM). The production rate was measured with the injections of oligomycienc and otenone-antimycine A. All chemicals were purchased from Agilent Technologies. All data were normalized to the cell number.
TIL04#9 cells were labelled with MitoTracker Green and cocultured with MEL04-MitoDsRed cells for 3 days. The cells were stained with an anti-USP30 conjugate or an anti-USP30 polyclonal antibody.
Mitochondria were isolated from a number of cells following a MitoCeption protocol using a Mitochondria Isolation kit. The Jurkat/Rho0 cells were immediately incubated with 2,020g for 15 min after the mitochondria were added. Four times a week this procedure was repeated. The Jurkat/Rho0 cells were added to with the EV-Entry system which was used to remove the EVs immediately after they were isolated. The cells were cultured for 6 weeks, and this procedure happened every 5 to 7 days.
TIL04#9 cells were cocultured with MEL04-MitoDsRed cells for 14 days, and DsRed– cells and DsRed+CD3+ T cells were sorted. 100 ng of totalRNA was then reverse-impregnated into a cDNA using the Prime-ScriptRT master mix and the RNeasy Plus Mini kit. PowerUp SYBR Green master mixes were used in real-time qPCR. Each sample had its Ct calculated as an internal control for BNIP, ATF7, ACEF6, CX CL8 andIL1B. The Ct for DsRed– cells was compared to the TIL03#9 cells. Supplementary table 5 has the primers listed.
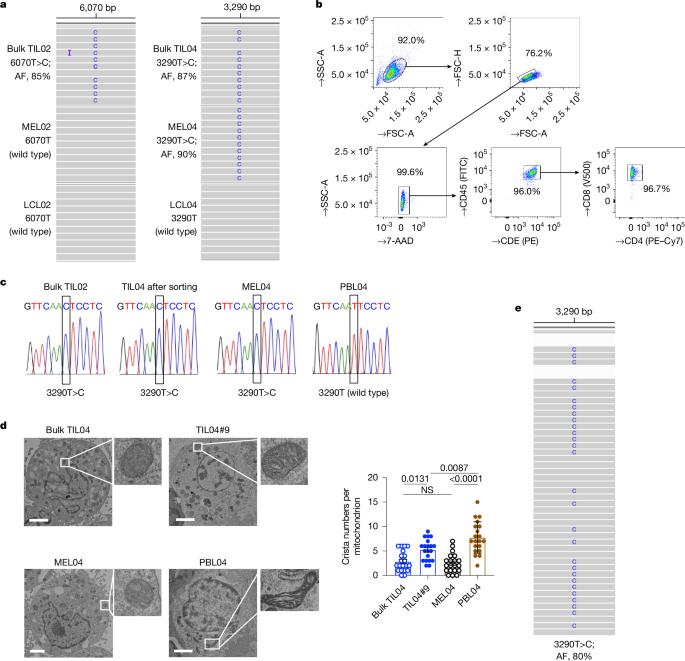
We cocultured wild-type mtDNA TIL04#9 or TILc03#5 cells with melanoma (MEL02-MitoDsRed, wild type; MEL04-MitoDsRed, mutated; MELc03-MitoDsRed, mutated) or breast cancer (MCF7-MitoDsRed, wild type; MDA-MB-231-MitoDsRed, mutated) cells for 14 days and subsequently sorted the TILs according to DsRed expression. The TILs were sorted by the type, as follows: DsRed+TIL04#9/02 wild type These TILs were then analysed.
The MitoCheck Activity Kits were purchased from the Cayman Chemical. The Mitochondria Isolation kit for cultured cells was used according to the instructions of the manufacturer. The manufacturer tells us to use the activity buffer with the isolated mitochondria, which is what we did. At 25 C, reactions were conducted using a microplate reader at the Central Research Laboratory and readings took for 15 minutes.
We used the DCFDA/H2FDA Cellular ROS Kit to screen for ROS. In brief, PBL04 cells and MEL04 cells were incubated with 20 μM DCFDA solution for 30 min at 37 °C in 5% CO2. Next, we extracted and purified EVs from the cells and analysed them by flow cytometry with a PS Capture Exosome Flow Cytometry kit (Wako) to create EV-conjugated beads.
Source: Immune evasion through mitochondrial transfer in the tumour microenvironment
Coculture of Red Cells with TfR-OVA (TfR = Tfr, Puro) and pVL-MitoDsRed (MEL02, MELc03, MCF
We obtained the data from the Genomic Data Commons data portal for patients with melanoma and from the USCS Xena database. USP30, USP33 and USP35 expression data in tumour tissues were used.
2 105 MEL03- MitoDsRed cells were put into a 35-mm glass-BOTTOM culture dish 24h before being allowed to adhere. The following day, coculture with 1 × 106 TIL04#9 cells labelled with MitoTracker Green was initiated. We began taking pictures with the digital hologram microscope every 30 min after 24 hours. The images were analysed with the help of software named “fiji”.
TIL04#9 and MEL04 cells were labelled with MitoTracker Green (Thermo Fisher Scientific) and MitoDsRed, respectively. Red cells were cocultured with labelled 1 106 TIL03#9 cells in a 35-mm glass- bottom culture dish for 2 days and then observed by a laser microscope. The cells were labelled as TIL 0.04#9 with a virus-conjugated monoclonal antibody for CD45
pcDNA3-TfR-OVA (Addgene, 64600)66, pBABE-puro (1764)67 and pVL-MitoDsRed (44386)68 were purchased from Addgene. The TfR-OVA cDNA was cloned into a pBABE-purovector according to the manufacturer’s instructions. The pBABE-puro-TfR-OVA was transfected into the cells with the help of a Lipofectamine 3000 reagent. The pMD2 was transfected with several plasmids (pRRE, Addgene, 12251, and pRSV-Rev, Addgene, 12253). The supernatants were concentrated and transduced into various cell lines after 48 h. The cells were named MEL02.MitoDsRed, MEL03MitoDsRed, MELc03MitoDsRed, and MCF7MitoDsRed. Cell lines from the same company were also generated.
The cells were immersed in 0.1 M PBS, with 2% Paraformaldehyde and 2%glutaraldehyde, for 16-18 h. Post-fixes were done with 2% osmium tetroxide. The specimen was embedded in a low-viscosity resin after washing with PBS. Subsequently, 80-nm-thick sections were prepared using an ultramicrotome (EM-UC7; Leica) and stained with uranyl acetate and lead citrate. The specimen was observed with a transmission electron microscope. We counted and quantified the number of cristae per mitochondrion.
Source: Immune evasion through mitochondrial transfer in the tumour microenvironment
Growth and growth of fibroblast-free digested tumour cells in a medium supplemented with Human Interleukin-2 incubated in RPMI1640
The cells were used after confirmation that they were free of mycoplasma, which was assessed using a Mycoplasma Detection kit from the manufacturer.
To establish cancer cell lines, 1 × 107 digested tumour cells were cultured in RPMI1640 medium containing 10% FBS (Cytiva), 1% penicillin–streptomycin (PS) and 1% amphotericin B (Thermo Fisher Scientific). Tumour cells were passaged at approximately 80–90% confluence and used when free of fibroblasts and proliferating beyond the tenth passage. To establish and expand cultured TILs, tumour digests were incubated in RPMI1640 medium supplemented with 10% human AB serum, 1% PS and recombinant human interleukin-2 (rhIL-2: 6,000 IU ml–1, PeproTech) in a humidified 37 °C incubator with 5% CO2. Half of the medium was aspirated from the wells and replaced with fresh complete medium and rhIL-2 every 2–3 days.
Source: Immune evasion through mitochondrial transfer in the tumour microenvironment
An Empirical Study of EAGLE Variant Allele Frequency in Melanoma Using Haplogrep Classification and Performance Assessment
After pooling the EAGLE results from all samples, we used z scores determined from the variant allele frequency (VAF) per position as a metric to evaluate potential variants. Alterations with 3 scores, VAF and read depths greater than 100 were selected to minimize false positives. The VaFs that were considered as polymorphisms were excluded from final reporting because they wouldn’t exhibit high VAFs due to the presence of a large number of non-cancerous cells. The Haplogrep uses Kulcyznski classification mode with 17_FU1 and 10 top hits to classify individual cases and identify possible haplo groups. If a variant is labelled hotspot or local private, it is considered to be a polymorphic variant and will be visually inspected to exclude possible errors at the termini of PCR amplicons or in homopolymer sequence stretches. We compared the presence of true variants, confirmed through a matching analysis of both normal and tumour samples, with the ones called only from tumour samples for performance assessment. True variants were called with a false negative rate of 12.2% and a false positive rate of 0, using these criteria.
The protocol for this study was approved by the appropriate institutional review boards and ethics committees of Yamanashi University Hospital, Chiba University Hospital, Shinshu University Hospital, Okayama University Hospital, Kindai University Hospital and Saitama Medical University International Medical Center. The study was done in line with the principles of the declaration.
Ten patients with melanoma, one with breast cancer and one with skin cancer were part of this study, and samples were used to establish TILs and matched cancer cell lines. Participants had undergone surgical resection at Yamanashi University Hospital, Chiba University Hospital, Shinshu University Hospitals or Saitama Medical University International Medical Center. The tumour tissues were processed as previously described60. In brief, surgically resected samples were enzymatically digested with 0.1% collagenase, 0.01% hyaluronidase and 30 U ml–1 deoxyribonuclease (Sigma-Aldrich) in RPMI1640 (Thermo Fisher Scientific) at room temperature. The digested tumour cells were subjected to filtration and density-gradient separation before use. Peripheral blood mononuclear cells were obtained from donated blood and through Ficoll–Uropoline density-gradient centrifugation. All participants gave their consent in writing.