Hydrogen has a hydrological footprint
Hydrogen and Oxygen Production from Offshore Electrolysers: Impacts of Desalinated Seawater on the Environment and Offshore Wind Energy
Given that hydrogen production and water use are inextricably bound, it is unlikely that water supply will continue to be omitted. The inconvenient truth, say both Brouwer and Rosa, is that solar energy is the cheapest source of low-carbon power available to produce hydrogen, but the regions with the best solar resources are also some of the most parched.
Another innovation that might benefit from using water for hydrogen production is the possibility to operate electrolysers offshore. Over the past year, teams in China and Europe have deployed platforms combining desalination equipment and electrolysers. The hope is that the floating plants will be able to operate reliably during storms and other assaults to offshore hardware, and thus cut the cost of offshore wind energy. Shipping hydrogen through pipelines is generally cheaper than moving the equivalent amount of energy through electrical transmission lines, and hydrogen proponents are betting that this rule will hold for passing energy from offshore wind farms back to land.
Lhyfe is investigating whether the oxygen from offshore electrolysis can counter the decline in dissolved oxygen in the ocean. In July of this year, researchers predicted that the volume of severely hypoxic zones would be reduced by 2% from the deployment of offshore wind farms. They also reported some surprising regional impacts. In the Indian Ocean’s Bay of Bengal, they projected that oxygen injection could enlarge the hypoxic zone.
The hydrogen producer Lhyfe’s 1-megawatt pilot platform operated offshore for 5 months this year using desalinated seawater, and a 10-megawatt platform is planned for Belgian waters in 2026. Lhyfe would like to mitigate the impact of desalinated water by avoiding chemical Additive in its treatment process and using brine with extra sea water.
Researchers with the municipal water authority Sydney Water and the University of Sydney, Australia, estimated in 2022 that integrating electrolysers into wastewater treatment plants could save the city about US$1.5 million per year 5. They calculate that the city’s 13.7 gigalitres per year of unused effluent could yield 0.88 megatonnes of green hydrogen per year — one-tenth of the amount Australia and New Zealand are expected to produce in 2030, according to analysts S&P Global in New York. Following further purification, Sydney Water says the viability of hydrogen and oxygen production using its treated water is confirmed.
Some observers, however, foresee potential environmental dividends if hydrogen producers tap seawater and waste water. Thomas Adisorn, a political scientist at Germany’s Wuppertal Institute for Climate, Environment and Energy, sees potential for projects such as that of Plug Power to improve the environment by supporting international development. “Putting more effort into using recycled waste water in developing countries that are exporting hydrogen could raise their capacity to build wastewater infrastructure,” says Adisorn, who organized a meeting in 2022 to help officials from water-scarce Jordan who were planning its hydrogen economy.
Using water presents a huge amount of potential, but also some really bad environmental impacts. The heated brines of some desalination plants can get into the sea and kill or destroy marine life. The 2020 review states that the most important impact of these plants is osmotic shock to marine organisms when super-salty brines cause their cells to dehydrate. The Red Sea, the Mediterranean and the Persian Gulf are some of the locations where organisms are most at risk. Nearly half of the world’s desalination capacity is concentrated in the Persian Gulf.
In many cases, hydrogen producers might be able to avoid adding strain to potable water supplies by tapping polluted or salty water, instead of potable water. Municipal waste water, waste water from oil and gas production, and even seawater are some of the options. The cost of water treatment and desalination plants are high, but the investment is low compared to the cost of hydrogen production.
Every kilogram of H2 needs 9 litres of H2O and 15 litres of water for purification.
The ending of the story is something that is still going on. If renewable energy that powers the process is included, there could be a lot more water use. The operation of solar panels and wind turbines might not consume much water, but manufacturing them does. The water footprint of green hydrogen is increased by 11 litres because of manufacturing a wind turbine. And the manufacture of today’s leading variety of solar power adds a huge 124 litres, mostly from the fabrication of silicon photovoltaic wafers.
Wallace’s Water Problem: What Have We Learned and How Do We Get There, and What Will We Learn in the Next Generation of Scientists?
The famous joke was made by David Foster Wallace and involved a fish telling another fish how the water is. The second fish asks what the hell is water.
That is a question nature faced when putting together the collection of articles about the substance that all life on Earth depends on. If you want to discuss the full spectrum of scientific and social issues associated with water, you have to address something of importance. Nature likes a challenge. The stories that are included in this supplement show how water affects the health of our civilization.
But only so far can you go with conservers. Rivers, wells and artificial reservoirs provide ample supplies for much of the world, but arid regions still struggle. Some researchers in these water-starved regions are turning their attention to the wet sponge that is the planet’s atmosphere. Fresh water could be obtained from thin air and water scarcity could be reduced.
No matter how abundant the supply, of course, water intended for drinking also needs to be clean and free of contaminants. Among the most pernicious are the ‘forever’ chemicals known as PFAS: chains of carbon and fluorine atoms held together by some of the strongest chemical bonds in nature, and impervious to most attempts to break them down. But engineers are devising various methods to crack them apart and purify PFAS-contaminated water.
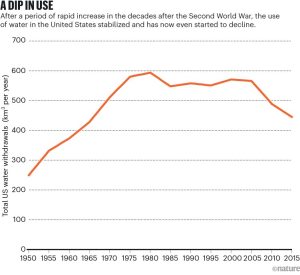
Source: Water: a source of life and strife
The FII Implementation of the Multi-Wavelength Discrepancies in the Theory of Large-Scale Structure
The Outlook is produced with the financial support of theFII Institute. Nature is in charge of all editorial content.