The FDA approves the first gene-editing treatment for humans
Casgevy: a CRISPR medicine for fetal hemoglobin in the U.S. by modifying the gene
The University of California, Berkeley professor, who was one of the first to use the new technique to modify genes, said in an interview that she was ecstatic. “It’s an exciting day and the beginning of a new day in medicine.”
bone marrow transplant can cure some patients, but most can’t find a suitable donor. About 20,000 patients in the U.S. have the severe form of the disease the CRISPR treatment would initially be used to treat.
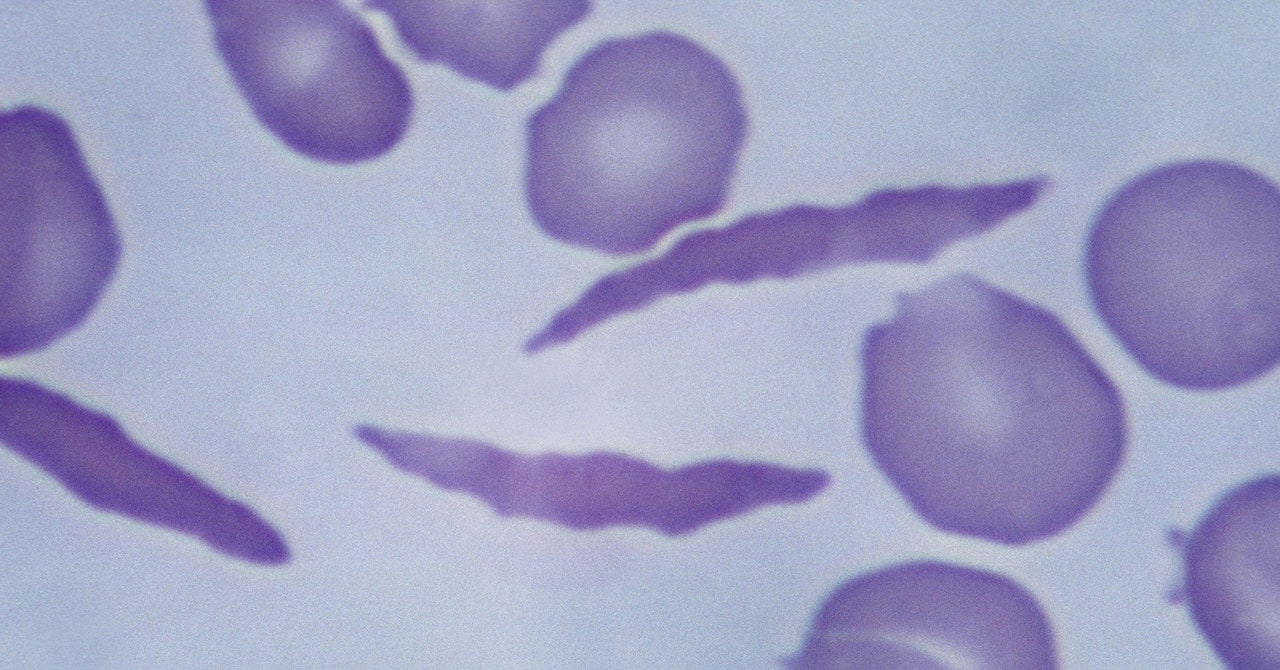
The edited cells produce a form of hemoglobin known as fetal hemoglobin, restoring normal function of red blood cells. While not a cure for the disease, the hope is the therapy, brand name Casgevy, is designed to be a one-time treatment that will alleviate symptoms for a lifetime.
The data presented to FDA showed that at least 85% of the subjects resolved their pain crises with the treatment. For patients with a related condition, the treatment produced similar results.
How to Make the Most of Your Life With Gene-Edited Blood Genes: A Case Study in Sickle Cell Disease (The Case of Casgevy)
Both of them cost a lot. Vertex said the wholesale price for Casgevy will be $2.2 million. Bluebird set the wholesale price of Lyfgenia at $3.1 million.
The treatments also require a complicated, arduous procedure that many hospitals are not equipped to provide. Patients may find treatment difficult to follow and hard to do.
Gene-editing, which allows scientists to modify the basic building blocks of life more quickly than ever before, is being studied as a treatment for illnesses like cancer, heart disease, diabetes, AIDS and Alzheimer’s.
The abnormal hemoglobin is responsible for carrying oxygen in to the body. The problem arises from a mutation in the HBB gene. Everyone has two copies of the gene—one from each parent. Both parents have a copy of the mutated genes for the children with the disease.
Like many sickle cell patients, Gray was forced throughout her life to repeatedly rush to the hospital for powerful pain drugs and blood transfusions. She couldn’t finish school, hold jobs, or care for herself or her children.
Gray’s has been able to start working full time at Walmart and spend more time with her four children because of the treatment.
I’ve had a new beginning since getting the treatment. Most of all, I no longer have to fear dying and leaving my kids behind without a mother,” Gray says. “My life is limitless now. I’m full of energy. There is no pain for me. It’s a real transformation.”
An assistant professor at the University of Michigan, Creary has a rare genetic disease and she has mixed reactions to the news. The promise of this technology for those with sickle cell disease is something I am excited about. But as this technology comes to market it’s going to be really interesting to see the ways in which profit overtake social justice.”
The majority of sickle cells patients live in countries that don’t have enough sophisticated medical centers to offer the complicated treatment. Even in the U.S., the treatment may not be widely available, making it difficult to access.
Can a CRISPR-based treatment save lives and improve lifetimes of cancer patients with an inherited inherited type of high cholesterol?
It’s questionable if sufficient research had been done to detect the effects of the treatment that wasn’t meant to be, and that could potentially lead to long-term health problems.
If the treatment helps patients live longer, the companies will follow them for 15 years to see how long the benefits last.
CRISPR based treatments have also shown promise for treated a rare liver condition known as amyloidosis, as well as an inherited form of high cholesterol known as familial hypercholesterolemia.
The acting deputy director of the National Human Genome Research Institute at the National Institutes of Health says it’s a big deal. This could change individual lives and reduce the burden of pain episodes.

