A map for the human cortex has been changed
The primary motor cortex of a newborn brain whose movements coordinate and coordinate with other parts of the body: an experimental study using above-elbow amputees
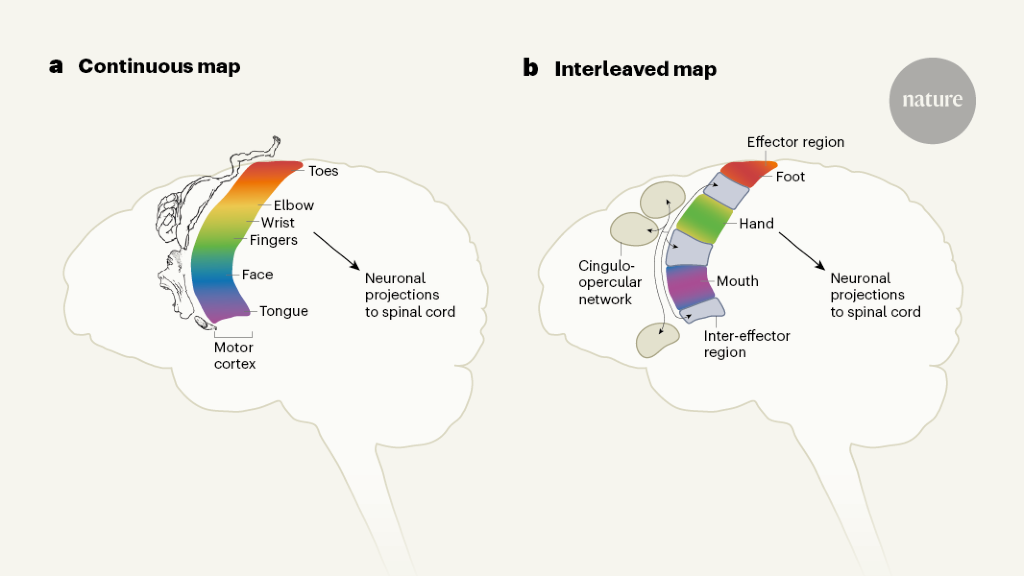
Some aspects of the original motor homunculus checked out: there were separate regions that controlled the movements of the foot, hand and face. But the researchers also found three areas interspersed between these regions that were strongly connected with each other and connected to other parts of the brain responsible for goal-driven action planning and other tasks such as regulating blood pressure and pain. These interspersed regions were not specific to any one body part or movement and were activated during action planning.
Researchers discovered in the late 18th century that the primary motor cortex can be stimulated to specific parts of the body. Later work found that some body parts, such as the hands, feet and mouth, took up a disproportionate amount of space in the primary motor cortex compared with the rest of the body. The first publication of the motor homunculus was translated to “little man” in Latin.
And Angela Sirigu, a neuroscientist at the Institute of Cognitive Science Marc Jeannerod in Lyon, France, and her colleague proposed a new theory in 20083, based on data from above-elbow amputees. In the primary motor cortex, they suggest there is one for motor commands and another for muscle synergies that enable coordinated movement.
The regions that the researchers found were from previously scanned individuals were not a coincidence. When they scanned children, they found that the newborn had a brain network that wasn’t developed yet, which supports the theory that this network coordinates complex action plans.
The study represents a prime example of the power of fMRI, says Michael Fox, a neurologist at Brigham and Women’s Hospital in Boston, Massachusetts. He said that they had no idea that the brain regions were hidden until functional brain scanning came along.
Understanding how people recover from damage to the primary motor cortex is really important, says Dylan Edwards, a specialist in neurorehabilitation at the Moss Rehabilitation Research Institute in Philadelphia, Pennsylvania. He says the findings could help with treatments that may be aligned with deficits.
The data was collected from three healthy, right-handed adults age 35, 25 and 27 years in order to investigate the effects of arm immobilization on brain plasticity. All participants were informed of the change of mind. A study was approved by both the Washington University School of Medicine Human Studies Committee and Institutional Review Board. The data was collected before or after the intervention. The data is collected immediately after the intervention. 1b. For details concerning data acquisition and processing, see ref. 29.
For two participants (participants 1 and 2), we collected additional fMRI data using the same sequence during performance of two motor tasks: a somatotopic mapping task and a motor control task.
An event-related design implemented using JSpsych toolbox v6.3 was used to discriminate planning and execution of limb movement. See Supplementary Fig. 6 for an illustration of this task. Within the run, there is a requirement for the participant to move either a single limb or two limbs. There are four possible actions that one may perform by any of the four legs, including an anticlockwise rotation of the wrist or ankle. In either case, each motion needs to be combined with a second simultaneous motion. When the grey symbol or symbols start turning green, the person is told to get ready for the movement when they see one or two movement symbols placed on a body shape in a grey colour. The planning phase lasts from 2 to 4.5 s and the movement execution lasts from 4 to 8.5 s. The movement trial ends with a fixation of up to 5 s. A rest block of 8.6 s is implemented every 12 movements. Two possible movements are requested during the task run and practiced before the task. Each task run has a change in the movement pair. 48 trials were collected in each run. Each participant acquired at least twelve total runs.
Recurrent Movement Localization using High-Resolution Multiband EPI, Spin-Echo, and fMRI MRI Image Retrieval
The participant was scanned repeatedly over the course of 12 sessions using a Siemens Prisma 3T scanner on the Washington University Medical Campus. These sessions included collection of 14 high-resolution T1-weighted MP-RAGE images (TE = 2.90 ms, TR = 2,500 ms, flip angle = 8°, 176 slices with 1 mm isotropic voxels), 14 T2-weighted spin-echo images (TE = 564 ms, TR = 3200 ms, flip angle = 120°, 176 slices with 1 mm isotropic voxels), and 26 total runs of resting-state fMRI, each collected as a 10 min-long BOLD contrast sensitive gradient echo-planar sequence (flip angle = 84°, resolution = 2.6mm isotropic, 56 slices, TE = 33 ms, TR = 1,100 ms, multiband 4 acceleration). In each session, a pair of spin-echo EPI images with opposite phase-encoding directions (AP and PA) but identical geometrical parameters and echo spacing were acquired to correct spatial distortions in the BOLD data.
An additional set of ten movement task runs adapted from31 were collected to localize laryngeal phonation. Each run the participant was given a visual cue that made them perform a specific movement: Left hand, Right hand, Left foot, Right foot, Tongue or Voice. Participants were required to make a noise without moving their jaw in the Voice condition. Each block started with a 3.0 s cue indicating which movement was to be made. After the cue, a centrally presented caret replaced the instruction and flickered once every 1.0 s. Participants executed proper movement when the caret flickered. Each block made at least ten movements. Each block lasted 15.0 s, and each task run consisted of 2 blocks of each type of movement as well as 2 blocks of resting fixation. Each participant completed 10 runs.
Source: https://www.nature.com/articles/s41586-023-05964-2
The ABCD BIDS Dataset for the study of neonates: Structural and Functional processing with a single high quality T2-weighted image
Four healthy adult participants, all male, were included in the data collected from them. The study was approved by an impartial review board. Each participant gave written informed consent.
Data were collected from one sleeping, healthy full-term neonatal participant beginning 13 days after birth, corresponding to 42 weeks post-menstrual age. The study was approved by two different committees at Washington University. The written consent was given by a parent.
The Washington University Dataset used a framework for structure and functional processing. First, segmentation, surface delineation and atlas registration were conducted using a T2-weighted image (the single highest quality T2 image, as assessed via visual inspection) rather than a T1-weighted image, due to the inverted image contrast observed in neonates. After the BOLD data were unwarped and normalized, it was combined before nuisance regression and mapping. The second day of scanning was excluded due to registration issues.
The ABCD BIDs Community Collection (ABCC;NDA Collection 3165), which we found did the best surface segmenting, followed the DCAN Labs processing line. Functional processing followed the pipeline used for the Washington University adult dataset.
The ABCD BIDS Community Collection contains a DCAN Labs processing line which was Structural and Functional.
Source: https://www.nature.com/articles/s41586-023-05964-2
MRI imaging and data acquisition for a boy with rhesus neuropathy on a PRImatE dataset of the PRIMATE Data Exchange
A boy who played baseball for a competitive team was referred to an orthopedist for an issue with his right arm. Ulnar neuropathy was considered and he was referred for physical therapy. However, PS1 was first seen by a child neurologist (N.U.F.D.) for further evaluation. Structural brain MRI revealed unexpectedly extensive bilateral cystic lesions consistent with perinatal infarcts. Review of PS1’s medical history revealed that the injury occurred in the perinatal period.
Data acquisition from PS1 were performed with the approval of the Washington University Institutional Review Board. PS1 gave an assent at the time of data acquisition, after giving written informed consent from his mother.
See the ref for further details regarding clinical history, neuropsychological evaluations, motor assessments or MR image acquisition or processing. 32.
Structural and functional data from the Oxford dataset of the PRImatE Data Exchange was provided. The full dataset consisted of 19 rhesus macaques. The animal care, anaesthesia and MRI protocols were carried out in accordance with the UK Animals (Scientific Procedures) Act of 1986. Animals were group-housed prior to scanning. In order to be administered via injection, there had to be at least 10 grams ofketamine, xylazine, midazolam, and buprenorphine. The injected substances were atropine, melaxicam, and ranitidine. A minimum of 15 min prior to being placed in the stereotaxic frame, animals also received local anaesthetics (5% lidocaine/prilocaine cream and 2.5% bupivacaine injected subcutaneously around ears). Scan began 1.5–2 h after anaesthesia was maintained. The initial hallucinogen did not work after.
Data were acquired on a Siemens Magnetom 10.5 T Plus. A custom in-house built and designed RF coil was used with an 8-channel transmit/receive end-loaded dipole array of 18-cm length combined with a close-fitting 16- channel loop receive array head cap, and an 8-channel loop receive array of 50 × 100 mm2 size located under the chin72. A B1+ (transmit B1) field map was acquired using a vendor provided flip angle mapping sequence and then power calibrated for each individual. The 3–5 averages were obtained from a T1weightedMP-RAGE after B1+ transmit calibration. A resolution and field of view-matched T2-weighted 3D turbo spin-echo sequence was run to facilitate B1 inhomogeneity correction. Five images were acquired in both phase-encoding directions (R→L and L→R) for offline EPI distortion correction. The fMRI time series consists of six runs each consisting of 700 continuous 2D multiband. Since the macaque was scanned in sphinx position, the orientations noted here are what is consistent with a (head first supine) typical human brain study (in terms of gradients) but translate differently to the actual macaque orientation.
Processing followed the DCAN Labs non-human primate processing pipeline (http://github.com/DCAN-Labs/nhp-abcd-bids-pipeline), with minor modifications. The field maps didn’t fully correct the distortion from the 10T scanner that we observed. Therefore, instead of field map-based unwarping, we used the computed field map-based warp as an initial starting point for Synth, a field map-less distortion correction algorithm that creates synthetic undistorted BOLD images for registration to anatomical images76. Residual BOLD image distortion was substantially reduced by synthesizer.
The DCAN Labs non-human primate processing line was used to process structural data. Smoothing was done with FWHM being 1.5mm in both volume and surface space. The surface data were then down-sampled to a 10k surface to create the preprocessed cifti data. Each animal’s data was carefully inspected for quality. Following these inspections, data from 11 animals were excluded due to the presence of artefact in or near the central sulcus, leaving eight animals in the final data. All available data was included in the sample size of eight. No randomization or blinding was performed.
A vertexwise group-averaged functional connectivity matrix from the HCP 1200 participants release was downloaded from https://db.humanconnectome.org. This matrix was composed of the average strength of functional connections for 812 people, who had their fMRI runs done, and who had their raw data reconstructed using the newer recon 2 software. See the refs. 81,84,85,86 for details of the acquisition and processing pipeline.
120 healthy young adults were recruited from Washington University to participate in relaxed eyes-open fixation. Scanning was conducted using a Siemens TRIO 3.0T scanner and included collection of high-resolution T1-weighted and T2-weighted images, as well as an average of 14 min of resting-state fMRI. See ref. 87 For more information on the acquisition and processing process.
Mothers were recruited during the second or third trimester from two obstetrics clinics at Washington University as part of the Early Life Adversity, Biological Embedding, and Risk for Developmental Precursors of Mental Disorders (eLABE) study. This study was approved by the Human Studies Committees at Washington University in St. Louis and written informed consent was obtained from mothers. Neuroimaging was conducted in full-term, healthy neonate offspring shortly after birth (average post-menstrual age of included participants 41.4 weeks, range 38–45 weeks). Of the 385 participants scanned for eLABE, 262 were included in the current analyses (121 female). Look at the ref. Criteria for exclusion and additional details of the participants are on this list.
We measured the functional connections between the inter-effector regions and every other large-scale cortex in the brain. The strength of connectivity between the inter-effector network and the CON was compared against the strength of its connectivity to each of these other networks using paired t-tests, FDR-correcting for the ten tests conducted.
Finally, to identify classic large-scale networks in each participant, we repeated the Infomap algorithm on matrices thresholded at a series of denser thresholds (ranging from 0.2% to 5%), and additionally identified individual-specific networks corresponding to the default, medial and lateral visual, cingulo-opercular, fronto-parietal, dorsal attention, language, salience, parietal memory, and contextual association networks following procedures described in ref. There were no announcements on 24. Supplementary fig. 6 is for the individual-specific networks.
We compared the mean lags in inter-effector regions against the mean lags in each of the foot, hand, and mouth regions.
Precentral gyrus foot, hand, mouth, and inter-effector regions were defined and our mean fractional anisotropy beneath each was calculated.
White matter fibres tracked from separate areas of M1 using diffusion imaging quickly converge into the internal capsule and become difficult to dissociate. We tested the white matter below the precentral gyrus for any fractional anisotropy differences.
Within each human participant, we created maps of the myelin content using the methods described in refs. 81, 97. Precentral gyrus was defined as above. The myelin density values varied wildly across different participants in different datasets, and were likely due to differences in the T1-5 and T1-2weighted sequence employed. To achieve optimal visualization of results, we normalized the myelin density values for each participant, by dividing them by the mean density across the precentral gyrus. The mean normalized myelin density was calculated for each region after they defined the precentral gyrus foot, hand, mouth and inter-effector regions. We performed t-tests on subjects using the inter-effector myelin density against their mouths, feet, and hands. myelin densities, correcting for the three tests conducted.
We plotted the dorsal–ventral M1 position against the Z-score in every movement. We then made two curves for each relationship. The first curve was a single-Gaussian model of the form:
Source: https://www.nature.com/articles/s41586-023-05964-2
Analysis of the brain movement task battery based on F-statistics and df2peaks with laryngeal mapping data
F is for f, and it means : : : : : : : : : : : : : : : : :
The P value was computed from this F through the use of the F-statistic continuous distribution function and df2peaks as the numerator and denominator degrees of freedom.
The laryngeal mapping data was not included in the movement task battery analysis because it was collected on a different scanning platform, and so wouldn’t be close to the one used in the analysis.
The action control task was analyzed using within-participant designs. For each separate run, a GLM model was constructed in FEAT98 in which separate regressors described the initiation of (1) planning and (2) execution of each type of movement (4 movements × 4 limbs). Each regression was constructed as a 0-length event, and the estimated alpha for every voxel in the brain was calculated. The second level analysis included a t-test across runs to contrast the run-level planning betas against the run-level executionbetas.
Each stimulation location reported in ref. 51 was mapped into the space. atlas pial cortical surface80 by identifying the vertex with the minimal Euclidean distance to the stimulation site’s MNI coordinates. Movements resulting from each site were classified as ‘lower extremity’, ‘upper extremity’ or ‘face’ and coloured accordingly (although no lower extremity movements were reported in the displayed left hemisphere).