The fight over abortion drugs
Predicting the Supreme Court Decision to Order Misoprostol, a Prescription for Abortion, Against an Unsound Oral Approach
Any abortion-rights dilemma is bound to incite religious and cultural passions, along with profound questions about a woman’s right to make the deeply personal decisions related to a pregnancy.
Dueling decisions in two federal district courts last week are likely to set up a showdown at the Supreme Court over the fate of the abortion pill mifepristone. If abortion opponents are successful, access to the pill — reportedly used in more than half of abortions in the United States — will be severely undercut.
Some of Kacsmaryk’s reasoning seemed plausible on its face: He noted that the agency used a process intended for serious illnesses and argued that pregnancy was not an illness. The drug was unsafe, he claimed in some parts of his ruling. It was a radical ruling, either way. There had never been a court that had given the go-ahead to approval of a drug.
The Court does not second-guess FDA decision-making lightly, according to his determination. But here, FDA acquiesced on its legitimate safety concerns – in violation of its statutory duty – based on plainly unsound reasoning and studies that did not support its conclusions.”
Acting last Friday soon after Kacsmaryk in the Texas-based FDA challenge, US District Judge Thomas Rice issued an order that would prevent the FDA from changing rules that would affect the availability of mifepristone in the 18 jurisdictions that were part of the litigation.
The officials want to be prepared in the event that the FDA will not approve the use of medication abortion, so they will still be able to do so. They are taking different approaches to the idea.
Supreme Court Justices will not decide “difficult moral and policy questions” related to abortion, as was written by the key fifth vote in favor of that decision.
“While California still believes Mifepristone is central to the preferred regimen for medication abortion, the State negotiated and purchased an emergency stockpile of Misoprostol in anticipation of Friday’s ruling by far-right federal judge Matthew Kacsmaryk to ensure that California remains a safe haven for safe, affordable, and accessible reproductive care,” Gov. Gavin Newsom’s office said in a release.
The decision from Kacsmaryk’s Amarillo courtroom more urgently drew the attention of Biden administration lawyers, drug manufacturers and abortion rights advocates.
He observed in a footnote that jurists often use the word “fetus” in opinions – as in fact the Supreme Court did in its Dobbs decision – but that he was favoring “unborn human” or “unborn child.”
DOJ argues that Mifepristone is better than Misoprostol in the United States than it is in Canada: “It’s a pill to take”
The medical groups that are challenging the FDA have no injury and offer baseless speculation, which makes them unable to file a lawsuit, according to DOJ lawyers.
Major adverse events, including major infections, blood loss, or hospitalization, occur in less than 3% of patients who take Mifepristone. There is almost no chance of death.
DOJ lawyers said that Kacsmaryk cherry- picked questionable materials to support his position, and doctors were quick to reject the conclusions of the judge.
She said that some facilities might be able to offer just one drug if they lost access to Mifepristone. She thought that it would lead to more unscheduled visits and emergency room visits for pain, incomplete abortions, and side effects.
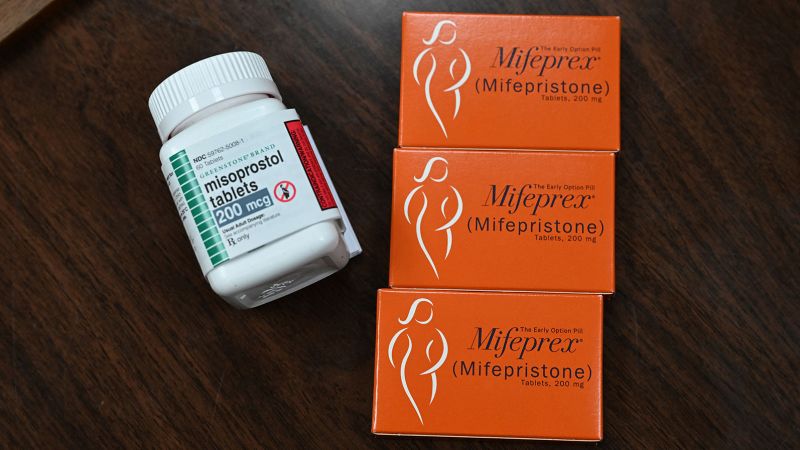
In the United States, the so-called abortion pill is actually two medications, mifepristone, sold under the brand names Mifeprex or Korlym, or known as RU-486, and misoprostol, which is taken about 24 to 48 hours later.
The two drugs work differently. Mifepristone blocks a hormone called progesterone, which the body needs for the pregnancy to continue. The uterus will expel its contents if there isn’t the hormone in it.
Misoprostol is approved by the FDA to prevent and treat gastric ulcers caused by nonsteroidal anti-inflammatory drugs (NSAIDs). It also is used off-label for other kinds of ulcers, and it has several gynecological uses, including to induce contractions, to decrease blood loss after delivery and to treat miscarriages.
A New Rule Change to the F.D.A. Order Formalization for the Use of Sublingually in the Treatment of Metamorphosis
“It’s a little old-school, but we could do it,” said Dr. Kristyn Brandi, an ob/gyn and abortion provider in New Jersey and a spokesperson for the American College of Obstetricians and Gynecologists.
A person who uses this method is instructed to use it sublingually, putting four pills under their tongue and leaving them there for 30 minutes, then swallowing what’s left with water. They can be vaginally used as well.
Typically, bleeding will begin within one to four hours after the first dose. Heavy bleeding usually lasts about three to five hours, but bleeding can last two weeks or longer, studies show.
People will have side effects from the more we give. And that’s not the goal. Our goal is to make sure that people respond to the medicine in a way that is effective, and also to make sure that they aren’t as uncomfortable as they could be. “Like I tell my patients, you do not need to suffer to have an abortion. That is not what this process is about.
Dr. Iffath A. Hoskins, president of the American College of Obstetricians and Gynecologists, says that although the one-drug option is safe, people shouldn’t have to make that choice because a lot of research shows that the two drugs in combination are highly safe and effective.
When clinically appropriate, we use off label medications, but because we have been using this for two decades, it’s not a valid option here.
The F.D.A lifted its requirement that patients get the drug from a provider after years of studies showed it would be safe. The drug can still be prescribed, but not by a doctor. For years before the rule was lifted, mifepristone was the only drug that the F.D.A. required to be obtained in person from a medical provider but that did not need to be taken in the presence of a provider — it could be taken at home or anywhere the patient chose. As a result, the F.D.A. and medical experts would dispute the judge’s contention that the 2021 rule change would create greater safety risks, especially since serious complications with mifepristone are rare.
Insights from State and Local Environmental Laws on Chemical Abortion: Arguments against the Wisconsin Supreme Court and the Court of Appeals
In six out of six referendums voted on by voters in the last eight years, they have sided with the majority of those who believe in abortion rights. Last week, they transformed the Wisconsin Supreme Court as a result of their support for abortion rights. A recent Ipsos poll found that nearly two-thirds of Americans want medication abortion to be kept legal.
Many anti abortion groups tried to undermine abortion by claiming that its harms girls and women and that abortion opponents wanted to protect pregnant people from the industry.
This strategy has helped to weaken abortion provider regulations, impose unnecessary regulations on clinics and make it difficult for women to have vaginal exams. State legislatures enacted burdensome laws requiring waiting periods before a woman can legally terminate a pregnancy. This all despite the fact that a woman is about 14 times more likely to die by carrying a pregnancy to term than having an abortion.
When the partial-birth abortion ban act was upheld by the Supreme Court, dubious science became more important to antiabortion advocates. In the case of scientific uncertainty, the court found that legislators had more latitude to regulate abortion. AntiAbortion groups could create their own data and have their own witnesses if they wanted to.
The scientific claims that are visible in lawsuits and congressional hearings are not completely accurate. Students for Life of America is asking the FDA to reconsider the approval ofMifepristone, a drug used to treat abortion-related bleeding, because of its negative effects on wastewater.
Women who have aborted a child — especially through chemical abortion drugs that necessitate the woman seeing her aborted child once it passes — often experience shame, regret, anxiety, depression, drug abuse and suicidal thoughts because of the abortion.
The theories of standing are dependent on layer after layer of speculation according to the defendants. But plaintiffs allege F.D.A.’s chemical abortion regimen “caused” intense side effects and significant complications for their patients requiring medical intervention and attention.
F.D.A.’s 2016 and 2021 changes thus significantly departed from the agency’s original approval of the abortion regimen. F.D.A. repeatedly altered its original decision by removing safeguards and changing the regulatory scheme for chemical abortion drugs.
The Comstock Act states that any article, instrument, substance, drug, medicine, or thing which is advertised or described in a way intended to lead another to use it or apply it for abortion is nonmailable. 18 U.S.C. § 1461 (emphasis added). It is clear that chemical abortion drugs are used to produce abortion. Therefore, federal criminal law declares they are “nonmailable.”
Comment on Kamcsmaryk’s Appeals Court Decision in the Case of Mifepristone: A Conservative Lawyer’s Perspective
“Adding regulatory uncertainty to the already inherently risky work of discovering and developing new medicines will likely have the effect of reducing incentives for investment, endangering the innovation that characterizes our industry,” the signatories write. Pfizer, for example, is a prominent drug company and has executives.
While we are not involved in this litigation, our focus is on ensuring the policy environment that supports the agency’s ability to regulate and provides access to FDA-approved medicines, said the Pharmaceutical Research and Manufacturers of America.
The lead counsel for Danco in this case said during a media press conference on 10 April that she was hopeful that either the Supreme Court or the appeals court would stop the decision before 14 April.
For that reason, even some conservatives criticized the decision. My colleague Adam Liptak wrote that the legal scholars he interviewed described Kacsmaryk’s decision as being of “poor quality” and “breathtaking sweep.”
Now that the filings have been submitted, the US 5th Circuit Court of Appeals Court could rule at any time on whether to put a hold on the order from US District Judge Matthew Kacsmaryk.
The FDA approval of medication abortion drugs was in jeopardy if the appeals court halted the judge’s ruling.
The Justice Department filing pushed back on the assertions by the challengers, made in their filing overnight in the emergency dispute, that the 5th Circuit did not have the authority to hear the appeal of Kacsmaryk’s ruling. The Justice Department also called out Kacsmaryk and the challengers for relying on anonymous blog posts to claim mifepristone is unsafe.
The state of Massachusetts and California have begun stockpiling mifepristone, and a federal appeals court has suspended a prescription for abortion drugs
Massachusetts Gov. Maura Healey and Washington Gov. Jay Inslee have announced that their states have begun stockpiling mifepristone in the event that access is disrupted. New York and California Gov. Kathy Hochul have claimed that their states are storing tens of thousands of prescriptions for the drug.
“Anti-choice extremists have shown that they are not stopping at overturning Roe, and they are working to entirely dismantle our country’s reproductive health care system, including medication abortion and contraception,” Hochul said. “New York will always be a safe harbor for abortion care, and I am taking action to protect abortion access in our State and continue to lead the nation in defending the right to reproductive autonomy.”
California said it shared the terms of its purchase agreement with other members of the Reproductive Freedom Alliance, a nonpartisan coalition of 21 governors who are committed to protecting reproductive rights, and who might also be interested in taking such action.
Washington Governor Jay Inslee mentioned last week that his state had bought a three yearsupply of the drug at the center of the ruling.
The state Department of prisons, which has a pharmacy license, was ordered by the governor to buy the drug by the end of March. The University of Washington also purchased 10,000 doses.
The University of Massachusetts and health care providers have taken action to make up for the shortage of the contraceptive in Massachusetts.
The manufacturer of the brand-name drug is saying that orders have increased significantly and are higher than they were a year ago.
Maine Gov. Janet Mills, who called the Texas decision “reckless” and a “fundamental assault on women’s rights,” said Monday that her administration is evaluating its options, “including procuring mifepristone if needed, to protect access to medication abortion for Maine women.”
The FDA approval of a medication for abortion drug was temporarily suspended by a Texas judge after a federal appeals court froze parts of his order.
The appellate order was handed down by Circuit Judges Catharina Haynes, a George W. Bush nominee, and Kurt Engelhardt and Andrew Oldham, both Donald Trump nominees. Haynes, however, did not sign on to some aspects of the order.
The judge deferred the question of whether the ruling should be frozen for more than a brief period of time to the judges hearing the case.
It’s unclear how the latest decision will interact with a ruling in a separate federal case in Washington state, filed by attorneys general from 17 states and the District of Columbia who are seeking to preserve access to the pills.
The motion filed by the Justice Department asks Rice to clarify his ruling, given that there appears to be tension with Kacsmaryk’s nationwide injunction.

