There are permanent vision problems after infections in people who use recalled eye drops
Interaction between EzriCare and Delsam pharma on over-the-counter eye drops: A case study in the United States
The two eyedrop companies recalled products in February but did not link the infections to them at the time.
The FDA said in February that Ezricare’s parent company, an India-based pharmaceutical provider named Global Pharma Healthcare, had failed to provide appropriate microbial testing of its over-the-counter eye product. The same could be said for Delsam pharma Artificial Eye Ointment, which was voluntarily recalled by the company.
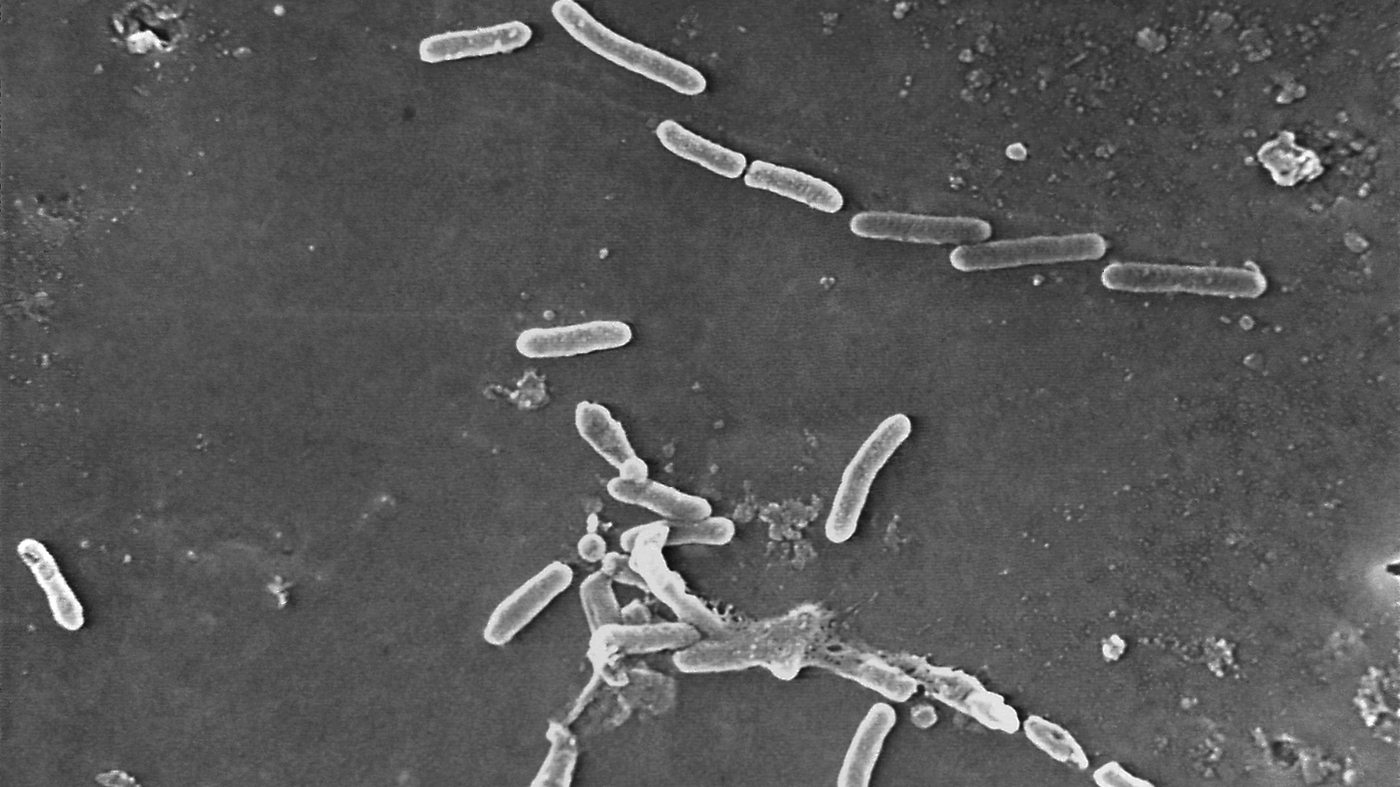
As of March 21, the CDC has identified 68 people in 16 states with infections of a rare strain of drug-resistant Pseudomonas aeruginosa never before reported in the United States. Most of the patients reported using artificial tears, the CDC said, and EzriCare Artificial Tears was the brand most commonly reported.
Allergic reactions include infections of the eye, respiratory tract, and urinary tract. There are four reports of eyeballs having to be removed and eight reports of lost vision.
“When we examined his right eye, there was a severe corneal infection,” said Dr. Marissa Shoji, a resident physician at the institute, who treated the patient. “He could only see shadows and was not able to see letters due to the extent of the ulcer.”
The infection came back in December. She says she went to the clinic every day, including Christmas Eve and Christmas Day. She was eventually diagnosed with permanent scarring of her eye.
The man went to the Bascom Palmer Eye Institute in Miami after a day of intense pain and vision problems in his right eye. He said that he had used artificial tears for eye dryness after no previous eye problems.
Montz, 72, had used EzriCare tears for about a week in November when she started noticing blurry vision. She woke up one morning and found a discharge on her pillow. Doctors found a large ulcer on her left eye at the emergency room.
“At some point, he was in danger of having permanent vision loss,” said Dr. Guillermo Amescua, an ophthalmologist at Bascom Palmer Eye Institute. He is 20 and has a scar on his eye, but with a transplant he might have a better chance of survival.
The antibiotic drugs that are designed to treat thebacteria are very much resistant to a particular variant of it. This type of pseudomonas had never been associated with an eye disease.
“In the long term, even after the infection is cleared, there’s a risk of needing a surgery such as a corneal transplant or other different types of surgeries to address scarring that may also significantly limit the vision,” she said.
The CDC says symptoms can include yellow, green or clear discharge from the eye; eye pain or discomfort; redness of the eye or eyelid; feeling like something is in the eye; increased light sensitivity; and blurry vision.
People should keep their eye drops out of their eyes. That means avoiding touching the tip of the bottle with their hands, skin or eye; not sharing the bottle with someone else; and not using expired products.
Infections are generally treated with antibiotics, but the bacteria are becoming increasingly resistant to multiple drugs. The CDC does not recommend patients undergo testing for infection unless they have symptoms.
An outbreak of eye infections in the United States, according to a South Carolina woman who has severe and permanent corneal scarring and eyes that have been dipped in oil
Renee Martray of South Carolina has severe and permanent corneal scarring resulting in vision loss. She says it’s like trying to peer through eyeglasses that have been dipped in oil.
The women said their problems began after they used EzriCare Artificial Tears, which is part of a US Centers for Disease Control and Prevention and US Food and Drug Administration investigation into a multistate outbreak of a rare strain of bacteria.
EzriCare said in a statement last month that when it learned of the investigation January 20, it was not aware of any testing that definitively linked the outbreak to its product.
Delsam Pharma’s product has not been linked to infections, but the CDC and US Food and Drug Administration have advised patients to stop using eye drops from either company pending additional guidance.
The investigation started with three distinct outbreaks and no apparent connection, said Dr. Maroya Walters, public health service officer and the CDC’s lead investigator on the outbreak.
The agency was initially notified in late June 2022 about eye infections among four people at an ophthalmology clinic in California, and tests revealed the unexpected bacteria behind them, Walters said.
Two more infections have been reported at long-term care facilities. There were 21 people involved in Connecticut in late July and three in Utah in August.
The patients had very little in common. They were from different kinds of facilities that had different kinds of infections,” Walters said. We looked at the products patients received but did not find a lot of similarities.
Oliva says she went to Bascom Palmer every day from August 4 to September 1 to receive topical antibiotics. They tried stronger and stronger drugs, but the infection persisted.
Doctors attempted to do a cornea transplant August 29, but the infection was too severe to save the eye. At the end of December it was replaced with a plastic implant.
Source: https://www.cnn.com/2023/03/24/health/eye-infection-patients/index.html
Keeping Eye Infections Alive: The Case of Oliva and Martray, a Patient who Cannot Walk Without a Helmet
“I cried constantly, asking why this happened to me. How could this have happened to me? I was searching for an answer: What happened to me? What is the moment? How? When? What did I do?” she told CNN in Spanish. “And to not have an answer, that is the most terrible thing.”
“I think without that, we never would have found this outbreak, and we need to really keep those programs strong, because I’m sure this is not the last time,” she said. To be able to detect future outbreaks, and to be even quicker at detecting them, we need to maintain these activities.
“On the other hand, I felt anger and indignation, because how could they be so negligent, so careless to make a product that could cost someone’s life?” said Oliva, who has filed a lawsuit against several companies including EzriCare and Global Pharma.
She says she had to relearn how to walk again due to stability issues. She can’t drive or do mundane tasks like going to the grocery store.
Martray, who has also filed a lawsuit against several companies including EzriCare and Global Pharma, said she learned about the bacteria from news articles her daughter sent her.
Source: https://www.cnn.com/2023/03/24/health/eye-infection-patients/index.html
When the boxes are sealed, how you put them in your eyes, and you lose them, but you don’t lose them: The case of EzriCare
She said she was shocked because she has always just trusted. “I feel that if they’re made for the eyes and the box is sealed and everything, it’s good. I never thought that something like that could happen. Bottles were sealed. You just grab them, you put them in your eyes, you don’t even think twice about it. You haven’t been wearing contacts since sixth grade.
EzriCare and Global Pharma did not respond to requests for comment on the lawsuits or the ongoing investigation. CNN reached out to EzriCare on March 17.
Martray says she’s been having frequent migraines from straining her eyes and can no longer do many things she previously enjoyed, like baking or crafts.

